Figure 1.
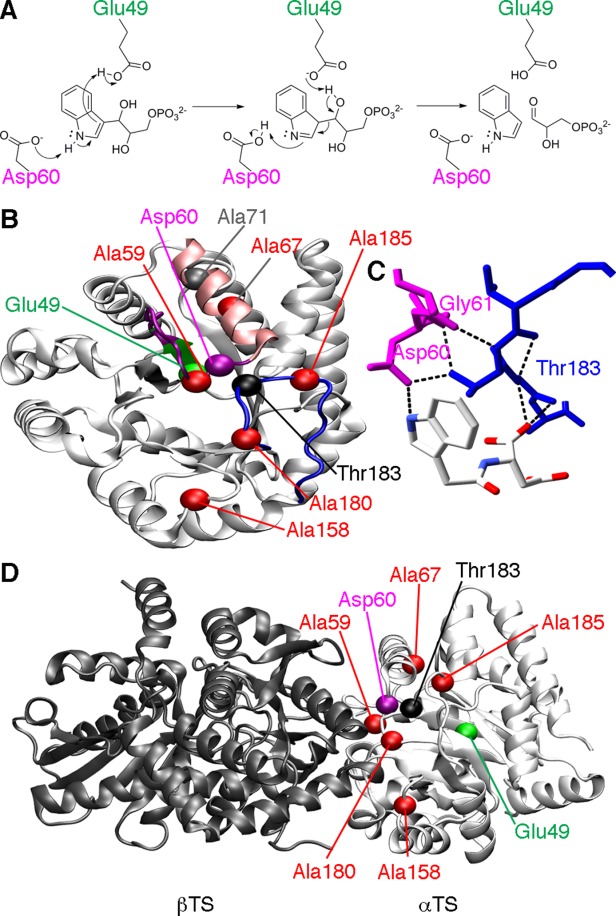
Catalytic function and loop interactions in αTS. (A) Chemical mechanism for αTS, highlighting the roles of Glu49 and Asp60 according to the step-wise mechanism.7 (B) Structure of αTS (PDB: 1K3U), showing the location of important amino acid residues, including Glu49 on the β2 strand (green), Asp60 on the β2α2 loop (magenta), and Thr183 on the β6α6 loop (blue). Also shown are the sites of the perturbations used to characterize the amino acid networks in E. coli αTS, including Ala59, Ala67, Ala158, Ala180, and Ala185 (red). It should be pointed out that in the absence of βTS, the α2' helix (pink) does not form, and instead the β2α2 loop is extended. Ala71 (grey), which is on the same amino acid network as Glu49 under some conditions, is also shown. (C) Close-up of the hydrogen bond interactions between the β2α2 (magenta) and β6α6 (blue) loops involving Thr183. The ligand shown is N-2[1H-indol-3-YL-acetyl]aspartic acid. (D) The locations of important amino acid residues in the context of the α−β TS dimer (PDB: 1K3U). Importantly, all of the NMR and kinetic studies reported here were performed on the αTS subunit alone, in the absence of the βTS subunit.
