Figure 2.
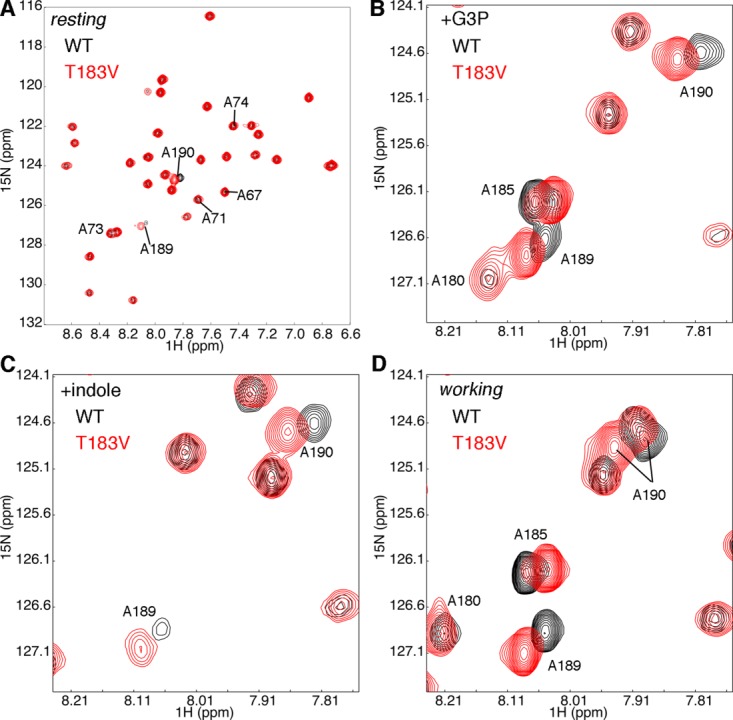
The T183V substitution leads to local changes in the β2α2 and β6α6 loops of αTS. Shown are comparisons of the 1H-15N HSQC spectra for WT (black) and T183V (red) αTS enzymes (A) without ligand, (B) in the presence of 10 mM D-G3P, (C) in the presence of 10 mM indole and (D) under dynamic chemical equilibrium conditions representing a 4:1 ratio of E:IGP to E:indole:G3P forms; these working state conditions are initiated with the addition of 10 mM D-G3P and 10 mM indole. Note that only the Ala residues are 15N labeled. NMR data were collected at 298 K on samples containing 0.5 to 1 mM protein in 50 mM potassium phosphate, pH 7.8, 2 mM DTT, 0.2 mM Na2EDTA, and 10% 2H2O.
