Figure 7.
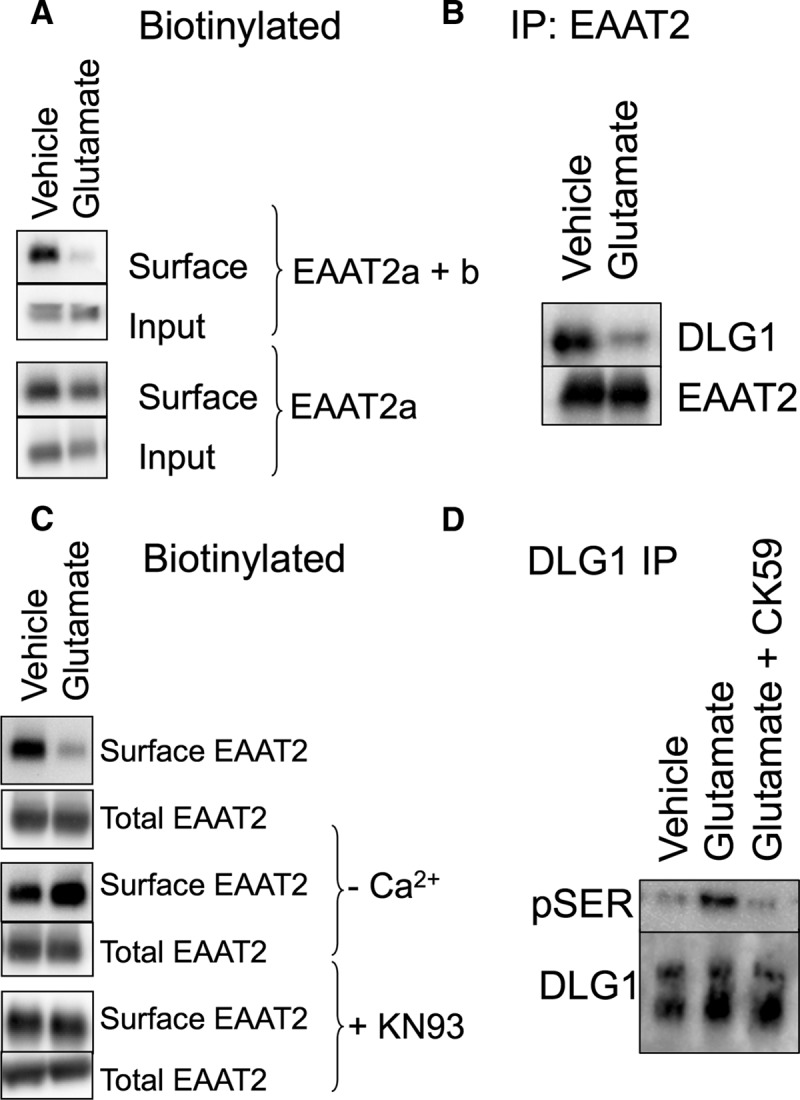
Endogenous EAAT2 in acute brain slices. A, Biotin-accessible EAAT2 protein from acute brain slices was isolated and analyzed by Western blot. An antibody that recognizes both EAAT2 isoforms (top two panels) indicates a decrease in surface localized protein in response to glutamate (54 ± 8.9% of vehicle control; p < 0.05 by t test; n = 5). An antibody that recognizes only the EAAT2a isoform, however, detects no significant change in the surface expression of the carrier in response to glutamate (bottom two panels; 95 ± 11% of vehicle control; n = 3), indicating that the change in biotinylated protein is due to a change in EAAT2b localization. B, Immunoprecipitation of EAAT2 with an N-terminally directed antibody coimmunoprecipitates DLG1, as also seen in the data presented in Figure 5C. Brain slices treated with glutamate display a decrease in the amount of DLG1 that coimmunoprecipitates with EAAT2 (26 ± 4%; p < 0.05 by paired t test; n = 4). C, The loss of biotin-accessible EAAT2 in response to glutamate in acute brain slices was blocked by the removal of calcium or the addition of the CaMKII inhibitor KN93 during the glutamate incubation. D, DLG1 was immunoprecipitated from vehicle- or glutamate-treated acute brain slices and probed with an antibody for pSer. Blots were quantitated by densitometry of the pSer bands (top) and normalized to the total DLG1 isolated by the immunoprecipitation (bottom). Glutamate treatment increases the pSer signal from DLG1 (141 ± 6% of control; p < 0.01 by one-way ANOVA compared to control; n = 4). The CaMKII inhibitor CK59 blocks glutamate-induced phosphorylation of DLG1 (104 ± 7% of control; n = 4).
