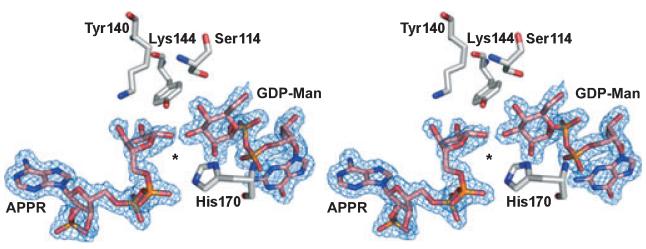
Fig. 8.
The RMD active site. Stereoview of the RMD active site showing the refined 2Fo–Fc electron density map around GDP-d-Man and APPR, contoured at 1.0σ. Ser114, Tyr140 and Lys144 form the catalytic triad. No clear electron density is seen for the nicotinamide ring, and even the nicotinamide ribose shows some indications of disorder; in fact, the average B-factor for the nicotinamide ribose is significantly higher than for either the adenine or guanine riboses. An asterisk is placed in the expected position of the disordered nicotinamide moiety. His170 is positioned in part of this ‘open’ space, a change of about 3.4 Å as compared with the position of the equivalent residue, His180, in P. aeruginosa GMD.