Abstract
Despite comprising 35% of transplants, the number of female transplant recipients has continued to decline. Accordingly, there is a growing attention to the issue of access to and outcomes of liver transplantation in women. The purpose of this review is to critically evaluate the published literature on etiologies contributing to gender-based disparities in liver transplantation focusing on the steps from chronic liver disease through transplantation including disparities in liver disease prevalence, access to liver transplant centers and transplant waiting list, receipt of liver transplantation once listed and disparities in post-liver transplantation outcomes. Our review finds factors contributing to this disparity may include gender differences in the etiology of underlying liver disease and patient and physician referral patterns, lifestyle and health care, but also utilization of an imperfect organ allocation system based on the model for end stage liver disease score and donor-recipient liver size matching. The review also highlights the need for further research in the area of gender disparity in order to develop appropriate approaches to address it and to improve allocation of this precious resource in the future.
Keywords: Female gender, Liver transplantation, Creatinine, Model for end stage liver disease, Disparity
Core tip: Liver transplantation is a life-saving procedure for many patients with end stage liver disease therefore it is of utmost importance to ensure equity in its distribution. Recently, growing attention has been placed on the issue of gender disparity in access to and receipt of a liver transplant. Factors contributing to this disparity include important differences in the etiology of underlying liver disease and indications for liver transplant that differ by gender. Systematic bias against women also appears to exist in many of the crucial steps of organ allocation. Better understanding of those mechanisms and their solutions are needed to improve liver transplantation rates in women.
INTRODUCTION
Chronic liver disease is the 12th leading cause of death in the United States with steadily increasing prevalence rates[1,2]. Notwithstanding medical therapies targeted to treat complications of end-stage liver disease, liver transplantation (LT) is the only life-saving procedure and is the treatment of choice for selected patients. In spite of the many improvements in liver organ allocation following the adoption of the model for end-stage liver disease (MELD) score in 2002, thousands of patients die awaiting liver transplantation every year as the supply of organs remains overwhelmed by the more than 17000 individuals on the waiting list. This burden is disproportionally exemplified in women, who comprised 35% of transplant recipients in 2013[3]. The proportion of female liver transplant recipients has continued to decline since the adoption of MELD in 2002. Data suggest that this proportion of women is also less likely to undergo liver transplantation once listed and have a greater probability of dying or becoming too sick to undergo liver transplantation compared to men[4]. Accordingly, there has been a fervid interest to identify the mechanisms behind gender discrepancies in disease burden and the process of liver transplantation (Table 1)[4-15]. The present review aims to evaluate the published literature on gender - based disparities in liver transplantation focusing on those areas of greatest significance to equity in LT, including the prevalence of chronic liver disease, access to LT, receipt of a liver transplant once listed and post-liver transplantation outcomes.
Table 1.
Characteristics of studies assessing the impact of gender on liver transplantation n (%)
| Topic | Ref. | Number of female patients (%) | Data collection |
| Access to LT Center | Bryce et al[5] | 66622 (46%) | Transplant Centers in Pennsylvania/UNOS |
| Access to waiting list and LT | Moylan et al[4] | 16262 (36%) | UNOS Database |
| Volk et al[6] | 19518 (39%) | SRTR | |
| Mathur et al[7] | 28759 (36%) | SRTR | |
| Renal function and MELD | Cholangitas et al[8] | 140 (38%) | Royal Free Hospital |
| Huo et al[9] | 103 (22%) | Taipei Veterans General Hospital | |
| Lim et al[10] | |||
| Mindikoglu et al[11] | 379 (45%) | Mayo Clinic | |
| 14530 (36%) | UNOS Database | ||
| Myers et al[12] | 14541 (36%) | UNOS Database | |
| Donor recipient size mismatch | Lai et al[13] | 12585 (36%) | UNOS Database |
| Mindikoglu et al[14] | 10741 (37%) | OPTN | |
| Post LT outcome graft/patient survival | Duffy et al[15] | 169 (58%) | Single Center UCLA |
UNOS: United network for organ sharing; SRTR: Scientific registry transplant recipients; LT: Liver transplantation; MELD: Model for end-stage liver disease; OPTN: Organ Procurement and Transplantation Network; UCLA: University of California, Los Angeles.
PREVALENCE OF CHRONIC LIVER DISEASE IN WOMEN
It is well established that etiologies of liver disease differ by gender. To determine an accurate epidemiology of chronic liver disease by gender in the United States has proven difficult however due to the lack of a national data collection system. Nonetheless, one can estimate prevalence rates by gender for individual diseases known to cause chronic liver disease. For example, women are 10 times more likely to have primary biliary cirrhosis than men and four times as likely to have autoimmune hepatitis[16,17]. Women are also more likely to present with alcohol and drug induced hepatotoxicity and acute liver failure as compared to men[18-20]. On the contrary, a National Health and Nutrition Examination Survey (NHANES) conducted between 2003 and 2010 found men to be significantly more likely to be chronically infected with hepatitis C virus than women[21]. Furthermore, gender also likely plays a role in disease progression for certain etiologies of chronic liver disease. Cross sectional studies have identified male sex as a risk factor for disease progression to cirrhosis by over 2.5 fold in patients with chronic hepatitis C[22]. Population prevalence of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) has been difficult to accurately establish[23]. Although the majority of studies based on the most recent NHANES data report NAFLD to be more prevalent in men than women, rates may vary depending on the diagnostic and staging tools utilized. Using data from the NASH Clinical Research Network (CRN), Younossi et al[2], reported that patients with biopsy proven NASH were more likely to be female than male while those using liver enzyme data or ultrasound to diagnose NAFLD report higher prevalence in men. Given the aging population, the increasing proportion of patients with NASH cirrhosis requiring LT, and the real possibility of curing most patients with chronic hepatitis C virus (HCV) liver disease, one would expect to see an increased demand for LT for women. The changing epidemiology of chronic liver disease along with the fact that disease severity may not be as accurately reflected in the MELD score for those diseases affecting primarily women makes the issue gender disparity in LT of growing concern.
GENDER DIFFERENCES IN CHRONIC LIVER DISEASE
The dominant mechanisms behind gender differences in the prevalence, natural history and outcomes of chronic liver disease (CLD) remains incompletely understood. Lifestyle choices regarding alcohol and intravenous drug use, as well as access to and use of medical care differ between men and women and may underlie some of the gender-based difference in CLD. Data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) showed that the prevalence of alcohol abuse and alcohol dependence is 2-3 times higher in men vs women[24]. However, while men consume and misuse alcohol at significantly higher rates, women experience shorter time intervals between the onset of alcohol use and alcohol related complications[25]. Hence it is postulated that the gender discrepancies in the prevalence, disease progression and outcomes of chronic liver disease may not all lie in behavior, but be largely influenced by the biological differences. For example, total body water, levels of alcohol dehydrogenase and slower rates of alcohol metabolism all contribute to the increased susceptibility of women to the toxic effects of alcohol[26,27]. In addition, differences in estrogen receptor concentrations, which in animal models is increased in male livers exposed to alcohol and unchanged in female alcoholic livers, may protect males from the alcohol induced liver disease seen in females[28]. On the contrary, female gender may play a beneficial role in viral hepatitis as studies have shown that estrogen can prevent stellate cell activation which is responsible for liver fibrogenesis[29]. The fall of estrogen is accompanied by a rapid increase in pro-inflammatory and anti-inflammatory cytokines[30]. Accordingly, the antifibrogenic role of estrogen may contribute to the discontinuous fibrosis progression in women compared to men with HCV as well as to why disease progression is significantly increased after menopause[31-33]. While the prevalence of NAFLD is higher in men than women of reproductive age, the protective effect of estrogen in NAFLD is eliminated after menopause[34]. Furthermore, after menopause, women have an increased risk of insulin resistance, hyperlipidemia and visceral fat, all of which are known risk factors for worsening NAFLD severity and could contribute to increased prevalence of NAFLD cirrhosis in women over time[35].
LIVER TRANSPLANT RATES: CHANGING PREVALENCE OF CLD
Chronic HCV and alcoholic liver disease together encompass the most common causes of cirrhosis, accounting for approximately one half of patients currently on the LT waitlist (Figure 1)[3]. NHANES data collected between 1988 and 2008 found that while the prevalence of alcoholic liver disease and HCV has remained stable over the past decade, the prevalence of NAFLD has steadily increased, reflecting the obesity epidemic[2]. Moreover, with continued strides in HCV treatment possibly leading to a subsequent decrease in liver transplantation rates for HCV cirrhosis and hepatocellular carcinoma (HCC), end stage liver disease secondary to NAFLD may change the spectrum of patients requiring liver transplantation. Moreover, as the prevalence of NAFLD is similar in post-menopausal women and men, women may have an increased representation on the LT waiting list in the United States in the near future.
Figure 1.
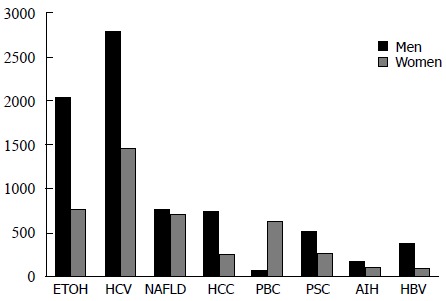
Primary cause of liver disease by gender, adult LT wait list candidates (2014). Based on Organ Procurement and Transplantation Network data as of July 1, 2014. Numbers of LT on the x-axis and etiologies of liver disease on the y-axis. PBC: Primary biliary cirrhosis; PSC: Primary sclerosing cholangitis; AIH: Autoimmune hepatitis; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; NAFLD: Nonalcoholic fatty liver disease.
LIVER TRANSPLANT REFERRAL
Gaining access to a life-saving liver transplant is a complicated and daunting prospect. Despite being chronically ill and even debilitated, patients must successfully navigate several challenging steps including first seeking medical care, referral to a hepatologist/gastroenterologist, referral to a transplant center for evaluation, placement on the liver transplant waitlist and finally receipt of liver transplantation. While women comprise just over 35% of candidates on the LT waitlist (Figure 2), challenges in establishing an accurate epidemiology of chronic liver disease by gender makes it unclear if this percentage reflects variations of disease burden in women or gender differences amongst those seeking and receiving healthcare. It is thus unclear how or if female gender plays a role in the first step of liver transplantation: referral to a transplant center.
Figure 2.
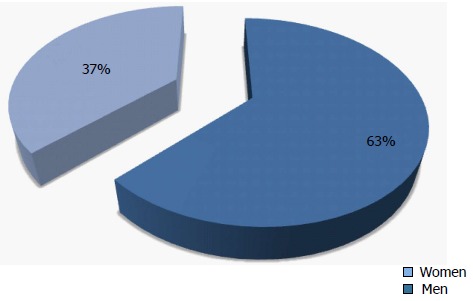
Adult Liver Transplantation Waiting List Candidates (2014). Based on Organ Procurement and Transplantation Network data as of November 28, 2014.
In the field of kidney transplantation, multiple studies have found that female sex is associated with a lower likelihood of inclusion on the transplant waiting list and suggest that gender disparity is not due to fewer women seeking health care or transplantation[36-38]. Thamer et al[39], in a national survey of nephrologists, found that men were more likely to be recommended for kidney transplant. Kucirka et al[40] found that women were more likely to be reported as unsuitable for kidney transplantation because of age or being medically unfit compared to their male counterparts. Unfortunately, information regarding LT referral patterns are limited. In one study from Pennsylvania, Bryce and colleagues found that with the exception of acute liver failure, the probability of being evaluated and listed for liver transplant was consistently lower for women[5]. The study was limited in that it did not assess individuals that may have been referred to a transplant center but never evaluated. Referral practice patterns are difficult to accurately assess in general and those of community physicians to liver transplant centers represent an even more troublesome and poorly studied area. Given the probable influence of physician variability in preferences and attitudes towards patient referral, screening and eligibility for LT, comprehensive evaluation of referral patterns are urgently needed to inform policies aimed at improving access to transplant centers for women.
LIVER TRANSPLANT RATES AND WAITLIST MORTALITY: INFLUENCE OF MELD
The current deceased donor liver transplantation (DDLT) organ allocation system in the United States is based on disease severity as measured by the MELD score as well as geographic proximity to available organs. Introduction and use of the MELD score in 2002 reflects the Institute of Medicine’s recommendation for an objective system to assess disease severity with less subjective criteria and less emphasis on waiting list time[41]. Prior to 2002, patients with end-stage liver disease awaiting DDLT were stratified based on subjective assessments of disease severity as well as their hospital status and accumulated time on the waiting list. While the major goal of the MELD score was to ensure liver allografts went to the sickest patients first, another goal was to eliminate possible systematic biases such as referral patterns as well as gender and racial disparities in organ allocation. Using a large national database of liver transplantation from the Organ Procurement and Transplantation Network (OPTN), Moylan et al[4] noted an increased gender disparity in LT after the implementation of MELD. This study found that women were more likely than men to die while waiting for LT or to become too sick for LT in the post-MELD era. In addition, women were also less likely to receive a liver transplant within 3 years of listing in the pre-MELD and post-MELD group. Mechanisms behind this disparity were not able to be determined from that investigation but it was speculated that the use of creatinine as a marker of renal function, donor recipient size mismatch and geographic disparities could all contribute to women receiving less liver transplants than men.
RENAL FUNCTION AND MELD
Creatinine is a breakdown product of creatine phosphate during muscle metabolism and is influenced by age, total muscle mass and laboratory variation[42]. As a small and freely filtered solute, creatinine is the most widely used marker in estimating glomerular filtration rate (GFR) and underlying renal function. Men tend to have higher levels of measured serum creatinine as they have a greater mass of skeletal muscle compared to women[42]. In end stage liver disease, decreased hepatic creatinine synthesis, increased tubular creatinine secretion and decreased skeletal mass contribute to falsely low serum creatinine even in the presence of renal impairment[43]. Although not validated in patients with liver disease, the Modification of Diet in Renal Disease (MDRD) formula, which estimates GFR using four variables (creatinine, age, ethnicity and gender), is superior to other formulas[44]. Using the MDRD formula, the estimated GFR in women is lower compared to men with the same serum creatinine, age and race. Therefore although serum creatinine does not reflect underlying renal function in women, this difference is not taken into account when calculating the MELD score, which is based on serum creatinine and not GFR.
Several studies have investigated the use of creatinine in the MELD score and whether it may result in a systematic bias against women. When comparing creatinine, estimated GFR, and respective MELD scores in men and women with end stage liver disease, Cholangitas et al[8] found that a 2-3 point correction was needed for women with MELD scores > 19 in order to make them comparable with men and accurately assess renal function and hence overall disease severity. Similarly, Myers and colleagues found that women with end stage liver disease were less likely to undergo LT and had a greater three month mortality than men despite having lower creatinine but worse renal dysfunction based on estimated GFR[12]. In order to determine whether the use of creatinine was the source of a systematic bias against women, the investigators of this study revised the MELD score to include estimated GFR. Interestingly, this did not improve the difference in 3-mo mortality in women compared to men. This discrepancy may be explained by the suboptimal accuracy of the MDRD formula in estimating GFR in patients with end stage liver disease despite its superiority to alternative formulas. Compared to the gold standard for evaluation of renal function with direct measurement of GFR with inulin clearance or iothalamate, only 67% of GFR estimates were within 30% of measured GFR amongst pre-transplant patients[45]. A study by Lim et al[10] using 125I-iothalamate for true GFR found that this was superior to creatinine in assessing mortality risk with a significant improvement of the discriminative ability when incorporated in the MELD score. Unfortunately, the prognostic impact was not evaluated between genders. Nonetheless, gender discrepancies in renal function and transplantation emphasized the inequities of the MELD score and stress the importance of examining alternative methods to evaluate renal function in patients with end stage liver disease.
DONOR-RECIPIENT SIZE MISMATCH
While data supports that renal function calculations and the use of creatinine in the MELD score contribute to decreased access to LT in women compared to men, other factors likely impact this disparity as well. An interesting study by Mindikoglu and colleagues investigated whether lower rates of LT in women vs men could be completely explained by MELD scores alone by comparing transplantation rates for each MELD score separately. They found that even within groups defined by MELD scores, women were generally transplanted at lower rates than men confirming that that suspicion that other factors play a role[11]. One of these factors is likely allograft size. Women are typically smaller than men in terms of total body mass as well as in liver size and overall height. It has been proposed that these differences lead to donor size mismatch and contribute to the gender disparity in LT[46].
Several published studies have looked at size differences and their contributions to this disparity. For example, Lai et al[13] used data from the OPTN to examine factors associated with differences in wait-list mortality between men and women in the MELD era and demonstrated that height, a surrogate marker for liver size, contributes to gender disparity. The investigators found that the majority of women were represented in the lowest quartile of the height distribution of wait-list candidates (< 165 cm). The mortality rates were 24% higher in this quartile and the rates of LT were significantly lower (P < 0.001). Again using OPTN data, Mindikoglu and colleagues found that size contributed to lower LT rates in women compared to men. Using median estimated liver volume and liver weights as surrogates for liver size, they found significantly lower volumes and weights in women men. After controlling for region, blood type and MELD score in a regression model including liver volume and weight, they found that women were significantly less likely to undergo LT than men suggesting that donor liver size mismatch significantly impacts gender disparity[14]. While the association between donor-recipient size and wait-list mortality remains poorly understood overall, a plausible explanation is that shorter and smaller women need smaller organs, which are preferentially offered to pediatric recipients. As a result, women may have to wait longer for a size appropriate allograft. This disparity in wait-list time may be reduced with the increased use of split-liver transplantation from deceased and living donors, but further research in this area is desperately needed.
GEOGRAPHIC DISPARITIES
Liver allograft distribution has historically been based on the geographic relationship between the hospital from which the organ is recovered and the transplant hospital in which the recipient is listed[47]. Unfortunately, this method of distribution has inadvertently created donor service areas (DSA) and regions with vastly discordant supply and demand ratios has contributed to significant geographic differences in access to liver transplantation[47]. Whether such geographic differences impact gender disparity in LT has therefore come under investigation. Mathur et al[7] evaluated this question using the OPTN regions with the largest sex-based disparities in transplant rates in the pre-MELD and MELD eras. They reported 16%-22% lower LT rates in women in regions 1, 2 and 10 during the pre-MELD era. In the MELD era, geographic disparity broadened with women exhibiting significantly lower LT rates in 6 of the 11 OPTN regions, with a maximum deficit of 35% in the Pacific Northwest (Region 6).
Despite a concerted effort by the United Network for Organ Sharing (UNOS) to change the liver allograft distribution policy, there has not been a significant reduction in the geographic variation in LT[47]. Broader sharing of organs has been proposed as a possible solution. The Share 15 policy, which ranks regional candidates with MELD > 15 higher than local candidates with MELD < 15, began in 2005 in the hopes of narrowing the range of median MELD at transplant among DSAs. As of yet, there are no studies investigating the effect of this policy in reducing gender-based geographic disparities. Nevertheless, as transplant needs and allograft supply differ widely amongst current regions, Gentry et al[48] proposed that broader sharing alone without redistricting may not be sufficient in reducing geographic disparities. But again, how redistricting affects women’s access to LT remains unknown. The OPTN/UNOS Liver and Intestinal Organ Transplantation Committee continue to investigate a number of approaches to reduce geographic disparities. Studies will also be needed to assess how these models affect gender-based disparities.
LIVER TRANSPLANTATION: OUTCOMES
Many factors contribute to outcomes after LT. The impact of gender on survival after LT varies with other donor and recipient factors such as race, age, ethnicity, as well as indication for LT. It also may vary by survival time and type of liver transplantation procedure performed. For example, female gender has been associated with advanced fibrosis and graft loss after LT for chronic HCV and this was increased with older donor age[49]. Long-term, women are reported to have improved survival than men however. In a small 20-year follow up study of LT recipients, women had improved survival over men[15]. One-year survival rates are similar between men and women with deceased donor liver transplantation, whereas they are increased compared to men in living donor liver transplantation[50]. The mechanisms behind these different survival rates by gender remain unclear.
Fortunately, the rates of re-transplantation continue to decrease. Women, however, continue to have slightly higher rates of re-transplantation compared to men as shown in one analysis of the 2009 Scientific Registry of Transplant Recipients[51]. The higher re-transplantation rate in women is speculated to be associated with the number of female candidates whose primary liver transplant was for diseases known to recur such as primary biliary cirrhosis, autoimmune hepatitis and NASH post-LT. Mathur et al[52] also found that female liver transplant recipients had 24% greater adjusted odds of receiving a low-quality liver allograft compared to their male counterparts which may affect both survival and re-transplantation rates. The etiology of the difference in quality of allografts for women recipients is unclear but felt possibly to relate to selection of shorter and older donors more often for women than men. Despite the utilization of poorer quality allografts in women, outcomes remain comparable across gender but warrant further longitudinal analysis as the epidemiology of LT changes.
CONCLUSION
LT is a life-saving procedure for many patients with end stage liver disease. Given its substantial and sustained improved survival rates over the last three decades, ensuring equity in its use remains crucial. Recent research has shed light on gender disparity that now exists in the LT process. The factors contributing to this disparity are many and include not only important gender differences in the etiology of underlying liver disease and patient and physician referral patterns, lifestyle and health care, but also utilization of an imperfect organ allocation system based on the MELD score and donor-recipient liver size matching which create bias against women at several steps. These biases include the use of creatinine as an imperfect marker of renal function in women, the need for smaller, less available liver allografts for the majority of women awaiting LT and geographic barriers to their distribution. This review highlights areas that require continued research to better understand such gender differences so that the disparity can be addressed and resolved. These steps will be of ultimate importance as we strive to improve the lives of all patients with end stage liver disease both pre- and post-liver transplantation.
Footnotes
Conflict-of-interest: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2014
First decision: November 19, 2014
Article in press: December 16, 2014
P- Reviewer: Peltec A, Ratnasari N S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530.e1; quiz e60. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Services Health Resources and Services Administration. OPTN/SRTR 2012 Annual Data Report [2012, cited 2014 August 18] Available from: http: //srtr.transplant.hrsa.gov/annual_reports/2012/pdf/00_intro_13.pdf.
- 4.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce CL, Angus DC, Arnold RM, Chang CC, Farrell MH, Manzarbeitia C, Marino IR, Roberts MS. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9:2092–2101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk ML, Choi H, Warren GJ, Sonnenday CJ, Marrero JA, Heisler M. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9:2113–2118. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 7.Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11:1435–1443. doi: 10.1111/j.1600-6143.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cholongitas E, Marelli L, Kerry A, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7:685–692. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 9.Huo SC, Huo TI, Lin HC, Chi CW, Lee PC, Tseng FW, Lee SD. Is the corrected-creatinine model for end-stage liver disease a feasible strategy to adjust gender difference in organ allocation for liver transplantation? Transplantation. 2007;84:1406–1412. doi: 10.1097/01.tp.0000282867.92367.d0. [DOI] [PubMed] [Google Scholar]
- 10.Lim YS, Larson TS, Benson JT, Kamath PS, Kremers WK, Therneau TM, Kim WR. Serum sodium, renal function, and survival of patients with end-stage liver disease. J Hepatol. 2010;52:523–528. doi: 10.1016/j.jhep.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147–1157. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54:462–470. doi: 10.1016/j.jhep.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant. 2010;10:2658–2664. doi: 10.1111/j.1600-6143.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19:89–95. doi: 10.1002/lt.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, Venick RS, Feist S, Goldstein L, Saab S, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652–661. doi: 10.1097/SLA.0b013e3181f5f23a. [DOI] [PubMed] [Google Scholar]
- 16.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 17.Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the united states. Hepatology. 2001;33:16–21. doi: 10.1053/jhep.2001.21165. [DOI] [PubMed] [Google Scholar]
- 18.Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WM, Squires RH, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 21.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 23.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:274–283. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Sutker PB, Tabakoff B, Goist KC, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18 Suppl 1:349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- 27.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 28.Colantoni A, Emanuele MA, Kovacs EJ, Villa E, Van Thiel DH. Hepatic estrogen receptors and alcohol intake. Mol Cell Endocrinol. 2002;193:101–104. doi: 10.1016/s0303-7207(02)00102-8. [DOI] [PubMed] [Google Scholar]
- 29.Bissell DM. Sex and hepatic fibrosis. Hepatology. 1999;29:988–989. doi: 10.1002/hep.510290351. [DOI] [PubMed] [Google Scholar]
- 30.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 31.Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villa E, Vukotic R, Cammà C, Petta S, Di Leo A, Gitto S, Turola E, Karampatou A, Losi L, Bernabucci V, et al. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One. 2012;7:e44624. doi: 10.1371/journal.pone.0044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 34.Baden R, Rockstroh JK, Buti M. Natural history and management of hepatitis C: does sex play a role? J Infect Dis. 2014;209 Suppl 3:S81–S85. doi: 10.1093/infdis/jiu057. [DOI] [PubMed] [Google Scholar]
- 35.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS. Sex inequality in kidney transplantation rates. Arch Intern Med. 2000;160:2349–2354. doi: 10.1001/archinte.160.15.2349. [DOI] [PubMed] [Google Scholar]
- 37.Ojo A, Port FK. Influence of race and gender on related donor renal transplantation rates. Am J Kidney Dis. 1993;22:835–841. doi: 10.1016/s0272-6386(12)70343-8. [DOI] [PubMed] [Google Scholar]
- 38.Bloembergen WE, Mauger EA, Wolfe RA, Port FK. Association of gender and access to cadaveric renal transplantation. Am J Kidney Dis. 1997;30:733–738. doi: 10.1016/s0272-6386(97)90076-7. [DOI] [PubMed] [Google Scholar]
- 39.Thamer M, Hwang W, Fink NE, Sadler JH, Bass EB, Levey AS, Brookmeyer R, Powe NR. U.S. nephrologists’ attitudes towards renal transplantation: results from a national survey. Transplantation. 2001;71:281–288. doi: 10.1097/00007890-200101270-00020. [DOI] [PubMed] [Google Scholar]
- 40.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12:351–357. doi: 10.1111/j.1600-6143.2011.03865.x. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Medicine (US) Committee on Organ Procurement and Transplantation Policy. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington (DC): National Academies Press; 1999. [PubMed] [Google Scholar]
- 42.Fauci AS, Lane HC. Harrison's principles of internal medicine. 17th ed. New York: McGraw Hill Professional; 2008. pp. 1137–1204. [Google Scholar]
- 43.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 44.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 45.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10:301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 46.Axelrod DA, Pomfret EA. Race and sex disparities in liver transplantation: progress toward achieving equal access? JAMA. 2008;300:2425–2426. doi: 10.1001/jama.2008.732. [DOI] [PubMed] [Google Scholar]
- 47.Redesigning Liver Distribution to Reduce Variation in Access to Liver Transplantation [2014,cited 2014 June 18]. Available from: http: //optn.transplant.hrsa.gov/ContentDocuments/Liver_Concepts_2014.pdf [Google Scholar]
- 48.Gentry SE, Massie AB, Cheek SW, Lentine KL, Chow EH, Wickliffe CE, Dzebashvili N, Salvalaggio PR, Schnitzler MA, Axelrod DA, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant. 2013;13:2052–2058. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai JC, Verna EC, Brown RS, O’Leary JG, Trotter JF, Forman LM, Duman JD, Foster RG, Stravitz RT, Terrault NA. Hepatitis C virus-infected women have a higher risk of advanced fibrosis and graft loss after liver transplantation than men. Hepatology. 2011;54:418–424. doi: 10.1002/hep.24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg CL, Steffick DE, Edwards EB, Heimbach JK, Magee JC, Washburn WK, Mazariegos GV. Liver and intestine transplantation in the United States 1998-2007. Am J Transplant. 2009;9:907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 51.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 52.Mathur AK, Schaubel DE, Zhang H, Guidinger MK, Merion RM. Disparities in liver transplantation: the association between donor quality and recipient race/ethnicity and sex. Transplantation. 2014;97:862–869. doi: 10.1097/01.tp.0000438634.44461.67. [DOI] [PMC free article] [PubMed] [Google Scholar]


