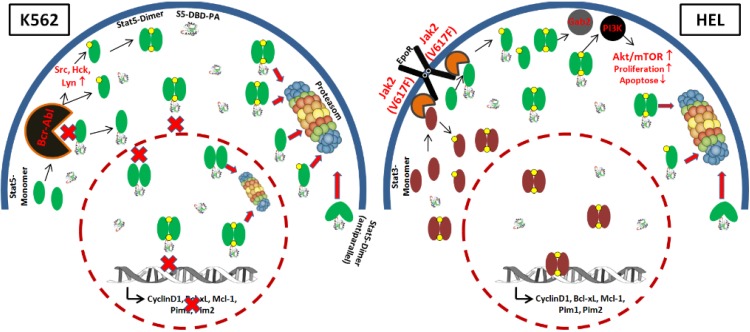
Figure 9.
Model for the Stat5-regulated survival mechanisms in Bcr-Abl-transformed K562 and Jak2(V617F)-transformed HEL cells and the inhibitory functions of S5-DBD-PA of cytoplasmic and nuclear Stat5. Oncogenic Bcr-Abl causes the phosphorylation of Stat5 in the K562 CML cells. This can happen directly or indirectly via the activation of Src family kinases: Src, Hck, Lyn. Activated dimers of Stat5 subsequently translocate to the nucleus and drive the expression of growth promoting and antiapoptotic target genes. The interaction of S5-DBD-PA with the DBD of Stat5 interferes with DNA-binding and transcription, blocks the nuclear im- and export of active dimers and reduces tyrosine phosphorylation (phosphate groups are indicated by a yellow point) potentially through steric hindrance of complex formation between the enzyme and the substrate. It possibly causes an enhanced degradation of Stat5. The erythropoietin receptor (Epo-R) associated activity of mutant Jak2(V617F) is characteristic of Epo-hypersensitive PV and acute erythroid leukemia diseases [36]. In the HEL cell line, Stat5 and Stat3 monomers are phosphorylated by oncogenic Jak2(V617F)+ independent of a S5-DBD-PA interaction. Active Stat5-dimer remain in the cytoplasm and promote essential PI3K-mediated survival pathways through cofactor interactions. The lack of nuclear Stat5 activity in HEL cells is accompanied by Stat3 activation and leads to a relatively higher resistance towards S5-DBD-PA when compared to K562 cells.