Figure 5.
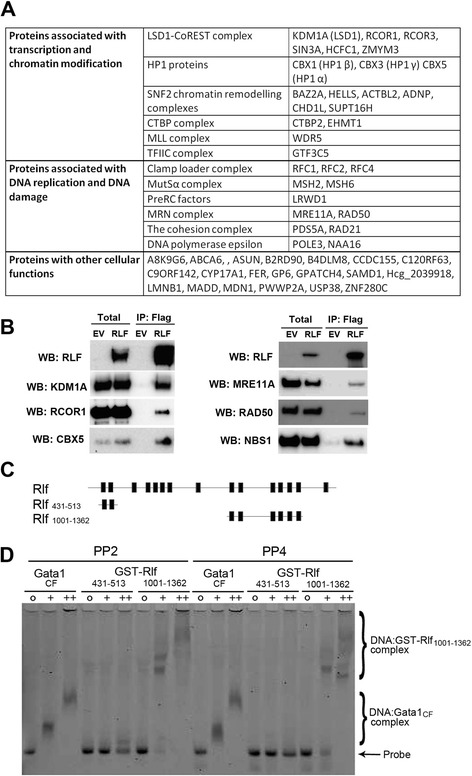
Rlf interacts with DNA and with proteins associated with transcription, chromatin modification and DNA replication and/or repair. (A) Summary of RLF interacting proteins determined by co-IP and mass spectrometry of HEK293T cells expressing RLF-Flag or an EV control (for details, see Additional file 9: Table S4). Proteins are grouped into categories and complexes based on previously reported functions. Three independent biological replicates were analysed per condition. Proteins classified as interacting partners were significantly enriched in RLF-Flag samples, p < 0.05, and are supported by at least five peptides. (B) Western blots showing co-IP of endogenous KDM1A, RCOR1, CBX5, MRE11A, RAD50 and NBS1 with exogenous RLF-Flag in transiently transfected HEK239T cells. Input represents 1% of the nuclear extract used for immunoprecipitation. Representative Westerns from at least three independent experiments are shown. (C) Schematic representation of mouse Rlf showing putative zinc finger domains. GST-fusion proteins used in EMSA experiments, Rlf431–513 and Rlf1001–1362, are also presented. (D) EMSA analysis testing the capability of GST-RLF fusions, Rlf431–513 and Rlf1001–1362, to bind to FAM labeled oligonucleotide probes, PP2 (lanes 4–9) or PP4 (lanes 13–18). Gata1CF, which is known to interact with DNA, was used as a positive control (lanes 1–3 and 10–12).
