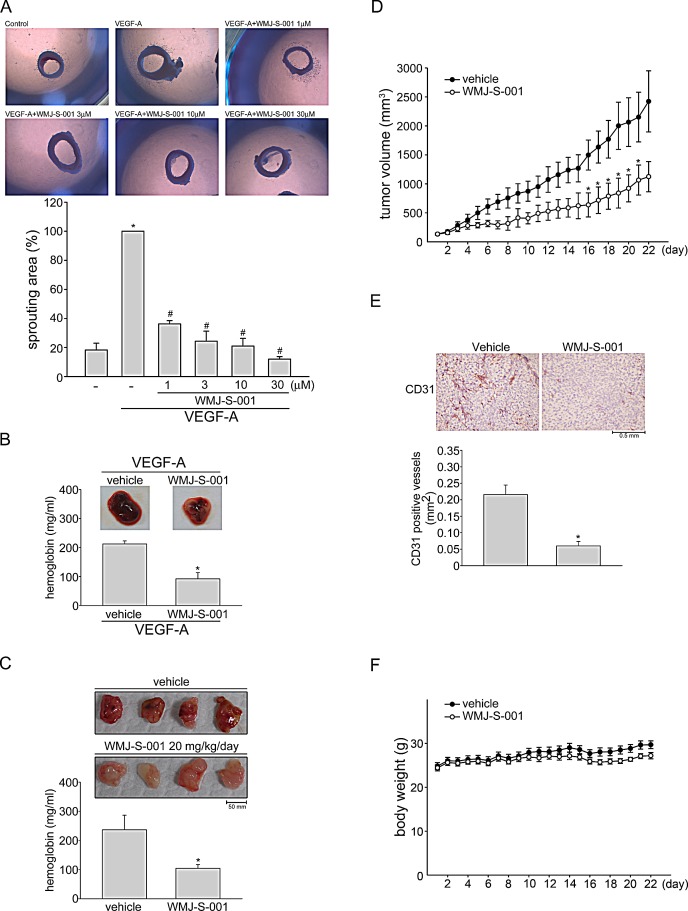
Fig.2. WMJ-S-001 inhibited angiogenesis and tumor growth in a mouse xenograft model.
Rat aortic rings were placed in Matrigel and treated with VEGF-A (20 ng/ml) in the presence or absence of WMJ-S-001 at indicated concentrations. The effect of WMJ-S-001 on formation of vessel sprout from various aorta samples was examined on day 8. Bar graphs show compiled data of average microvessels area (n=4). *p < 0.05, compared with the group treated with VEGF-A alone. (B) Matrigel mixed with VEGF-A was injected subcutaneously into the right flank of nude mice. After implantation, animals were treated intraperitoneally with vehicle or WMJ-S-001 (20 mg/kg/day) for 10 days. Matrigel plugs removed from the mice administered intraperitoneally with vehicle or WMJ-S-001 were shown. Hemoglobin levels in the Matrigel plug were quantified. Each column represents the mean ± S.E.M. of four plugs in each group (*p < 0.05 as compared with the vehicle-treated control group, n = 4). (C) HCT 116 colorectal cancer cells were mixed with Matrigel and then injected subcutaneously into the right flank of nude mice. After implantation, animals were treated intraperitoneally with vehicle or WMJ-S-001 (20 mg/kg/day) for 10 days. Matrigel plugs removed from the mice administered intraperitoneally with vehicle or WMJ-S-001 were shown. Hemoglobin levels in the Matrigel plug were quantified. Each column represents the mean ± S.E.M. of six plugs in each group (*p < 0.05 as compared with the vehicle-treated control group, n = 6). (D) Nude mice bearing xenografts of HCT116 cells were treated intraperitoneally with vehicle or WMJ-S-001 (20 mg/kg/day) for 22 days. The control group received vehicle only. Tumor volumes were calculated as described in the Materials and Methods section. Values represents the mean ± S.E.M. (*p < 0.05 as compared with the vehicle-treated control group, n = 5). (E) After 22 days of treatment, mice were sacrificed and the blood vessels in solid tumor sections were stained with anti-CD31 antibody. Images of immunohistochemical staining representative of eight randomly selected xenograft tumors (four tumors from each group) with similar results are shown. Compiled results are shown at the bottom of the chart. Each column represents the mean ± S.E.M. of four tumors from each group (*p < 0.05 as compared with the vehicle-treated control group). (F) No significant differences in body weights were found between the vehicle- and WMJ-S-001-treated groups.