Table 1.
Optimization study
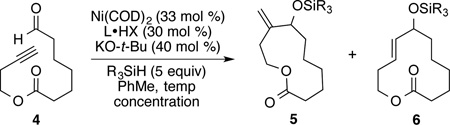 | |||||||
|---|---|---|---|---|---|---|---|
| entry | L•HX | temp (°C) |
R | C mM |
rra (5:6) |
NMRb yield % |
isolated yield % |
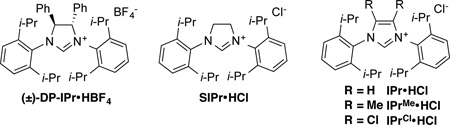
| 1 | DP-IPr | rt | Et | 3 | 93:7 | - | 21 |
| 2 | DP-IPr | 90 | Et | 3 | - | trace | - |
| 3 | DP-IPr | 90 | i-Pr | 3 | - | trace | - |
| 4 | SIPr | 90 | i-Pr | 3 | >95:5 | 27 | - |
| 5 | IPr | 90 | i-Pr | 3 | >95:5 | 18 | - |
| 6 | IPrMe | 90 | i-Pr | 3 | >95:5 | 35 | - |
| 7 | IPrCl | 90 | i-Pr | 3 | >95:5 | 67 | 66 |
| 8 | IPrCl | 50 | i-Pr | 3 | >95:5 | 30 | - |
| 9 | IPrCl | 90 | i-Pr | 5 | >95:5 | - | 69 |
| 10 | IPrCl | 90 | i-Pr | 10 | >95:5 | 32 | - |
| 11 | IPrCl | 90 | Et | 5 | 65:35 | - | 51c |
| 12d | IPrCl | 90 | i-Pr | 5 | >95:5 | 17 | - |
| 13e | IPrCl | 90 | i-Pr | 5 | >95:5 | - | 63 |
 | |||||||
Determined by 1H-NMR analysis of the crude mixture.
1,3,5-trimethoxybenzene was used as internal standard. Preparative experiments were not conducted with an internal standard due to complexity of removing the internal standard.
Combined yield.
Ni(COD)2 (23 mol %), IPrCl•HCl (20 mol %), KO-t-Bu (24 mol %).
Ni(COD)2 (43 mol %), IPrCl•HCl (40 mol %), KO-t-Bu (44 mol %).
