FIGURE 7.
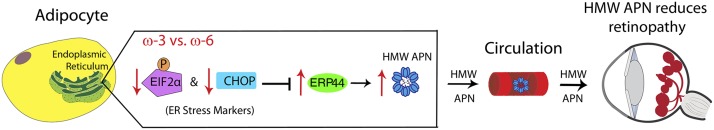
Schematic illustration of proposed pathway for ω-3 LCPUFA regulation of APN production via modulation of ER stress. In ER, activated pERK-EIF2α pathway (an unfolded protein response) in adipocytes upregulates a central transcription factor CHOP, leading to inhibition of ERP44 synthesis, an essential ER protein involved in APN assembly and secretion. Phosphorylation of EIF2α and CHOP upregulation are attenuated with ω-3 LCPUFAs compared with ω-6 LCPUFAs, thereby increasing ERP44 protein concentrations and accelerating APN assembly to a higher-order form and secretion from adipocytes into circulation. Increased serum higher-order forms of APN in turn bind their receptors adipoR1 and adipoR2 in retinal endothelial cells and macrophages, mediating protective effects of ω-3 LCPUFAs on retinal vasculature in retinopathy. APN, adiponectin; CHOP, C/EBP homologous protein; EIF2α, eukaryotic initiation factor 2α ER, endoplasmic reticulum; HMW, high molecular weight; LCPUFA, long-chain PUFA.
