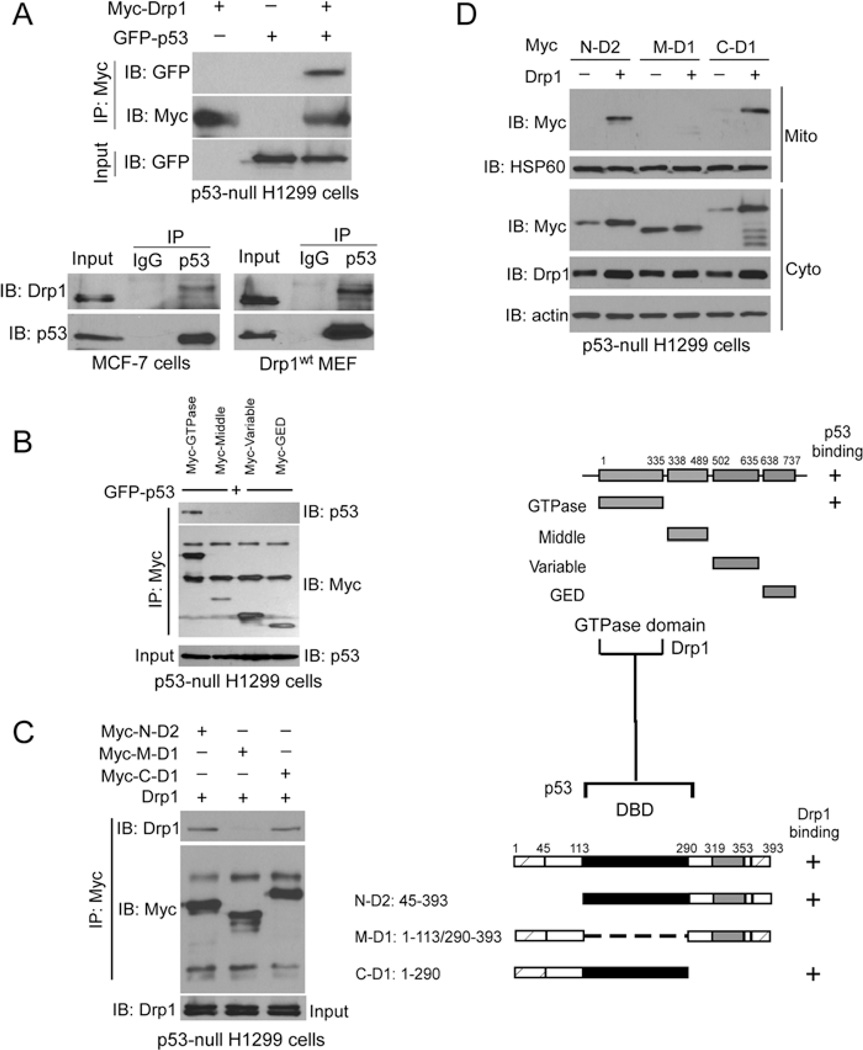
Figure 3. Drp1 binds to p53.
(A) Upper panel: GFP–p53 was co-expressed with Myc–Drp1 in H1299 cells. Immunoprecipitates (IP) with anti-Myc were immunoblotted (IB) with anti-GFP antibody. Lower panel: immunoprecipitates with anti-p53 in Drp1WT MEFs and MCF-7 cells were immunoblotted with the anti-p53 and anti-Drp1 antibodies. (B) Mapping analysis on the interaction between Drp1 and p53. Left: H1299 cells were expressed with the indicated Drp1 truncated mutants. Immunoprecipitates with anti-Myc antibody were determined by Western blot analysis using the indicated antibodies. Right: map of Drp1 truncated mutants. The region that interacted with p53 is labelled ‘ + ‘. GED, GTPase-effector domain. (C) Left: H1299 cells were expressed with a series of p53 deletion mutants, and immunoprecipitates with anti-Myc antibodies were immunoblotted with the indicated antibodies. Right: map of p53 truncated mutants. The region that interacted with Drp1 is labelled ‘ + ‘. The data suggest that the GTPase domain of Drp1 interacts with the DBD of p53. (D) H1299 cells were transfected with the indicated plasmids. Western blot analysis of mitochondrial and cytosolic fractions was carried out with the indicated antibodies. HSP60 was used as a mitochondrial loading control, and actin was used as a cytosolic loading control. IB, immunoblot; IP, immunoprecipitation.