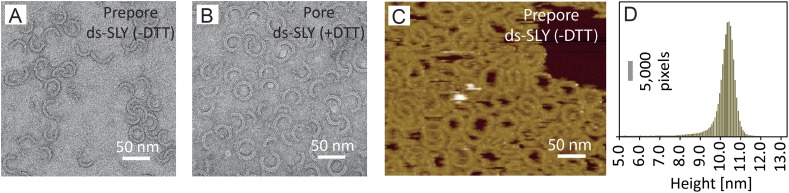
Figure 2. Negative-stain EM and AFM of disulphide-locked suilysin.
(A) Negative-stain EM disulphide-locked suilysin (ds-SLY) on egg PC:cholesterol monolayers (45:55%), locked in the prepore state (−DTT). (B) as (A), for disulphide-locked suilysin incubated in the presence of 5 mM DTT in solution to reduce the disulphide bridge, so that the suilysin is rapidly converted to the pore conformation. (C) AFM of densely packed suilysin prepores, confined to the egg PC-rich domain of a phase-separated egg PC:DDAB:Cholesterol (33:33:33%) supported lipid bilayer, with its corresponding height distribution (D) referenced to the membrane surface.