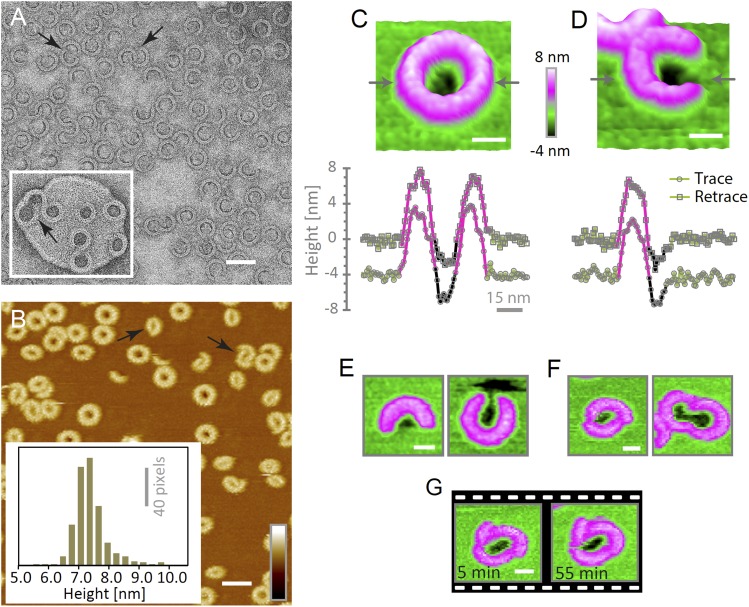
Figure 4. Suilysin assembles into ring- and arc-shaped oligomers that perforate the membrane.
(A) Negatively stained EM of arc- and ring-shaped assemblies of wild-type suilysin on an egg PC:cholesterol (45:55%) lipid monolayer, and (inset) on a liposome of egg PC:cholesterol (45:55%). (B) AFM topography of wild-type suilysin on a supported egg PC:cholesterol (67:33%) lipid bilayer. The wild-type suilysin extends 7–8 nm above the lipid bilayer background, as indicated by the height histogram for 402 individual particles (inset). (C) The AFM topography of a complete suilysin ring reveals a circular hole (dark) in its lumen, whereas the lipid bilayer surrounding the ring remains intact (green). (D) The topography of a suilysin arc shows a hole (dark) in the membrane only partially enclosed by the suilysin assembly. Images in C and D are shown in a 15° tilted representation, and height profiles measured across the ring/arc confirm membrane perforation. (E) Examples of wild-type suilysin arcs of different lengths. Transmembrane holes are consistently observed. (F) Examples of interlocked-arc assemblies. As shown in the right image, the membrane area removed by the two arcs is larger than the hole in the complete ring (C). (G) Sequence of AFM images of the same interlocked-arc assembly, stable for at least 50 min. Scale bars A–B: 50 nm, C–G: 15 nm, full z colour scale B–G: 12 nm.