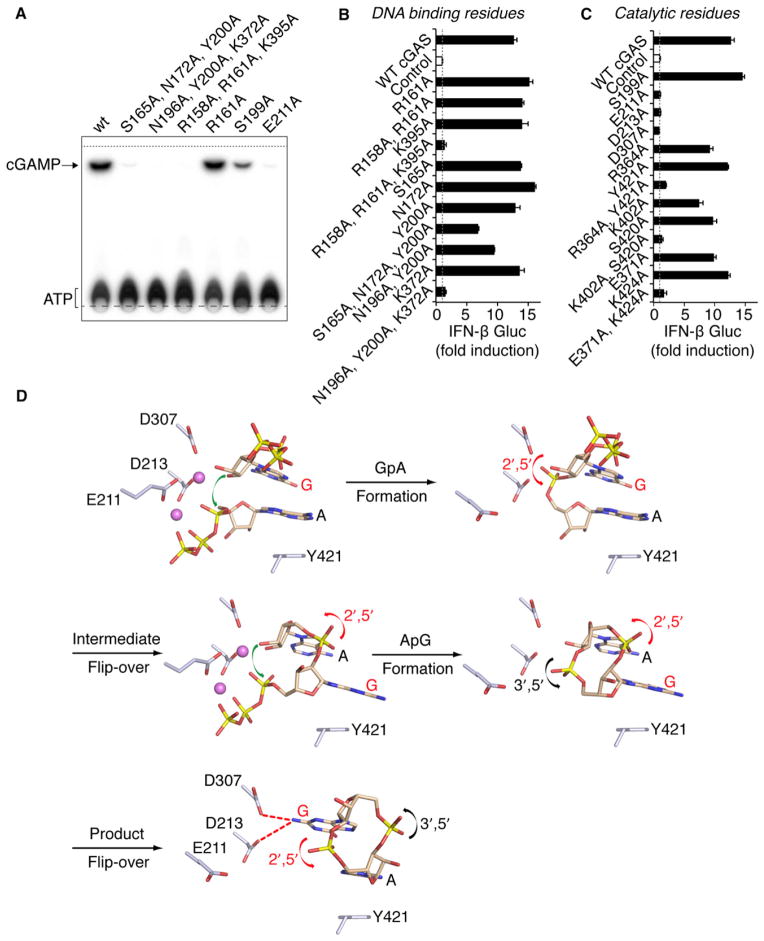
Figure 7. Functional Analysis of cGAS Mutants and Model for Two-Step Generation of c[G(2′,5′)pA(3′,5′)p].
(A) Levels of c[G(2′,5′)pA(3′,5′)p] formation by cGAS full-length WT and indicated mutants were compared by TLC analysis. Long- and short-dashed lines indicate the origin and solvent fronts, respectively.
(B and C) Expression vectors of murine cGAS WT or carrying single and multiple alanine mutations of DNA binding (B) and catalytic (C) residues were transiently transfected into HEK293 cells together with an IFN-β Gluc reporter and constitutive STING and Firefly luc expression plasmids. In this setting, expressed cGAS is engaged in the cytosol by the cotransfected DNA plasmids. Gluc values were determined in triplicate 36 hr after transfection, were normalized to Firefly luc, and are shown as fold induction over control plasmid (as mean ± SEM). Data in (B) and (C) are representative of three to five independent experiments for each mutant.
(D) A schematic representation of a proposed model associated with a two-step generation of c[G(2′,5′)pA(3′,5′)p] within the single catalytic pocket of cGAS. In this model, the first step involves formation of a 5′-pppGpA intermediate followed by formation of c[G(2′,5′)pA(3′,5′)p]. Note also that the bound ligand is predicted to undergo two flip-overs on the pathway to c[G(2′,5′)pA(3′,5′)p] formation.