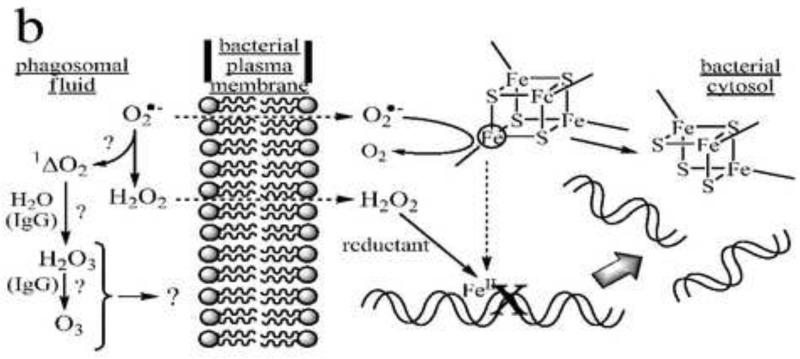
Fig. 1.
Four models of bactericidal action in neutrophils. Panel a: MPO-dependent oxidative killing. Granule-derived MPO catalyzes formation of HOCl from endogenous chloride and NOX-2-derived superoxide or hydrogen peroxide. HOCl and/or secondary chloramines formed by reaction with endogenous amines inactivate plasma membrane-localized proteins involved with energy transduction and biosynthesis (represented here as the F0F1-ATP synthase and a generic proton-symporting metabolite transport protein). Additional reactions of HOCl may involve formation of nitryl chloride (NO2Cl) if sufficient nitrite ion is present in the phagosomal fluid, and generation of hydroxyl and carbonate radicals following one-electron reduction by superoxide. Panel b: MPO-independent oxidative killing. Superoxide ion perfuses the bacterial membrane to react with iron-sulfur clusters in cytosolic dehydratases, releasing ferrous ion, which relocates to vulnerable target sites (here shown as genomic DNA). Subsequent site-specific oxidation by H2O2 in Fenton reactions leads to irreversible loss of function (here double-stranded cleavage of the DNA). (After Imlay and coworkers [53].) Additional (but presently controversial) pathways may involve singlet oxygen-initiated formation of hydrogen sesquioxide or ozone in antibody- or amino acid-catalyzed reactions. Panel c: Nonoxidative killing. Granule-derived cationic antimicrobial peptides (CAMP) aggregate in the bacterial plasma membranes to form membrane-spanning pores that dissipate ion gradients essential to homeostasis and energy transduction. In Gram-negative bacteria, granule-derived bactericidal permeability-increasing protein (BPI) binds to lipopolysaccharide (LPS), initiating a set of transformations that promote phospholipase (PLase) activation and access to and degradation of the bacterial membranes by these and other lytic proteins, including serum-derived complement (C7-C9) and group IIA-phospholipase A2 (PLA2). The latter gain access to the phagosome by binding to extracellular bacteria prior to phagocytosis. PG = peptidoglycan. (After Elsbach and coworkers [23,155].) Panel d: Redox-driven nonoxidative killing. Electrogenic NOX-2 respiration acts to polarize the phagosomal membrane, driving influx of electrolyte cations (here, K+). Hydrogen peroxide, formed as the respiratory end-product, is destroyed in catalatic reactions (here via MPO catalysis). The increased ionic strength causes release of granule-derived cationic seprocidins (e.g., cathepsin G, elastase) from anionic biopolymers, which then attack the bacterium. (After Segal and coworkers [41].)