FIGURE 1.
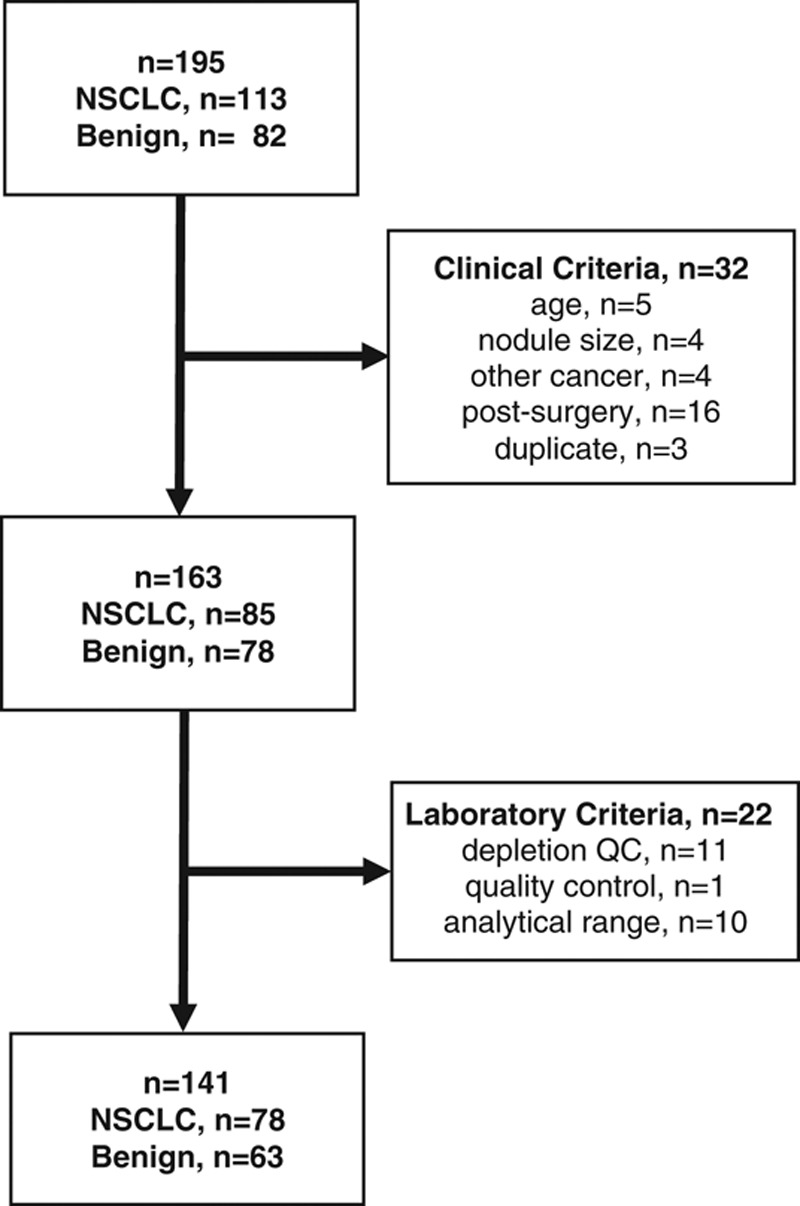
Flow chart of validation study plasma sample availability and exclusions based on clinical and laboratory criteria. The participating centers (n = 4) identified 195 plasma samples from 195 subjects initially satisfying the study inclusion criteria. After completion of clinical data monitoring, 32 candidate samples were excluded due to subject age less than 40 years; nodule size less than 8 mm or larger than 30 mm; nodule cancer pathology other than NSCLC; plasma sample collection after surgery; or provision of duplicate samples from the same subjects. An additional 22 samples were excluded after sample analysis based on laboratory criteria including depletion column quality control, mass spectrometry quality control, or a classifier result outside of the validated analytical range.21 A final total of 141 samples from 141 subjects satisfied the prespecified clinical and laboratory criteria, including 78 cancer and 63 benign samples, and were included in the data analysis.
