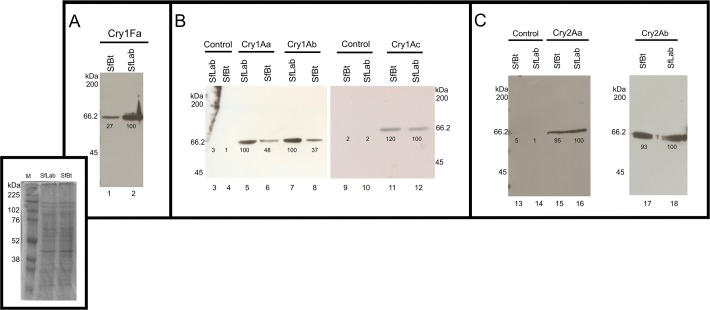
Fig 1. Analysis of binding of Cry toxins to BBMV from SfLab and SfBt populations.
Panel A, Binding of Cry1Fa: Lane 1, binding of Cry1Fa to SfBt; Lane 2, binding of Cry1Fa to SfLab: Panel B, Analysis of binding of Cry1A toxins: Lanes 3 and 10, control of BBMV from SfLab without toxin incubation; Lanes 4 and 9, control of BBMV from SfBt without toxin incubation; Lane 5, binding of Cry1Aa to SfLab; Lane 6, binding of Cry1Aa to SfBt; Lane 7, binding of Cry1Ab to SfLab; Lane 8, binding of Cry1Ab to SfBt; Lane 11, binding of Cry1Ac to SfBt; Lane 12, binding of Cry1Ac to SfLab. Panel C, binding of Cry2A toxins: Lane 13, control of BBMV from SfBt without toxin incubation; Lane 14, control of BBMV from SfLab without toxin incubation; Lane 15 binding of Cry2Aa to SfBt; Lane 16, binding of Cry2Aa to SfLab; Lane 17, binding of Cry2Ab to SfBt; Lane 18, binding of Cry2Ab to SfLab. Binding of biotinylated toxins (10 nM) to BBMVs from S. frugiperda SfLab and SfBt populations was performed as described in material and methods. Numbers under the bands represent the percentage of each band on the blot calculated after scanning optical density of the bands with ImageJ program and using one band of similar size in the gel as 100% reference. Insert: Gel 12% SDS PAGE with BBMVs prepared from SfLab and SfBt populations. Lane 1, Rainbow molecular marker (GE); Lane 2, 10 μg of BBMV SfLab; Lane 3, 10 μg of BBMV SfBt