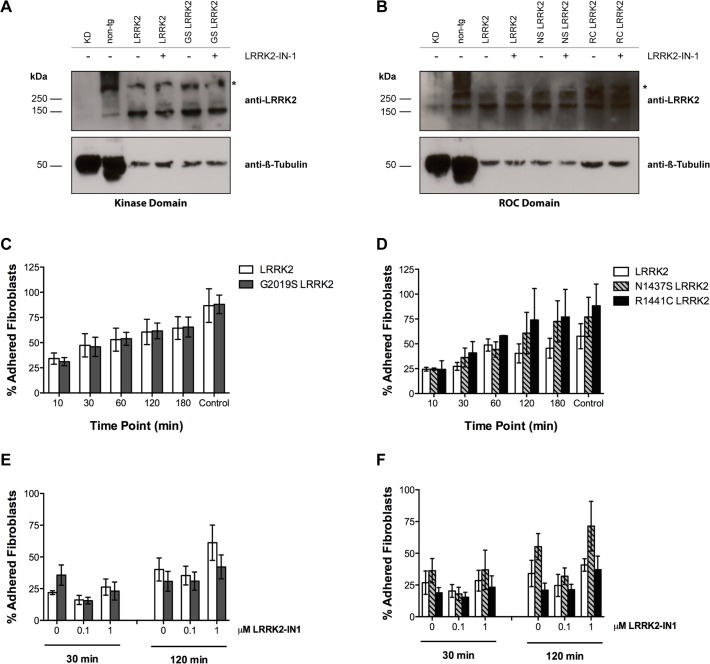
Fig 5. Treatment with the LRRK2-IN-1 kinase inhibitor does not alter adhesion properties in LRRK2 primary human skin fibroblasts.
A-B: Western blot analysis of LRRK2 expression in primary skin fibroblasts from healthy subjects (LRRK2) and PD-patients with mutations in the kinase (A) and ROC domain (B) of LRRK2 after treatment with vehicle or 0.1μM LRRK2 IN-1. The MJFF#2 antibody we used recognizes both human and mouse LRRK2. Brain lysates from a Lrrk2 knock-down mouse (KD) and cortex lysate from a non-tg mouse served as negative and positive controls, respectively. One representative fibroblast line per mutation group is shown and 30μg protein was loaded on a 7% acrylamide SDS-gel. C-D: Percentage of adhered fibroblasts with LRRK2 mutations in the kinase (C) and ROC (D) domain at different time points. No significant differences in adhesion capacity were observed between lines. E-F: Percentage of adhered fibroblasts with LRRK2 mutations in the kinase (E) and ROC (F) domain after treatment with vehicle control (0), 0.1μM or 1μM LRRK2-IN-1 for 30 and 120 minutes. No differences were observed in fibroblasts with LRRK2 mutations in the kinase (E) and ROC domain (F). Data represent mean ± SEM; n = 4 independent experiments (C, E) and n = 3 independent experiments (D, F). Healthy-Subjects (LRRK2) = 4 lines; G2019S LRRK2 patients (GS) = 3 lines; N1437S LRRK2 patient (NS) = 2 lines; R1441C LRRK2 patients (RC) = 1 line. (Two-way ANOVA with Repeated Measures).