Abstract
The enteric nervous system has been studied thus far as an isolated unit. As researchers probe deeper into the function of this system, it is evident that the neural network stretches beyond enteric neurons. It is formed by both intrinsic and extrinsic neurons innervating the gut, enteric glia, and innervated sensory epithelial cells, such as enteroendocrine cells. This Review series summarizes recent knowledge on function and disease of nerves, glia, and sensory epithelial cells of the gut in eight distinctive articles. The timing and growing knowledge for each individual field calls for an appropriate term encompassing the entire system. We call this neuronal ensemble the “gut connectome” and summarize the work from a food sensory perspective.
“Tell me what you eat, and I will tell you what you are,” wrote the French gastronome Jean Brillat-Savarin in 1826 (1).
Although at the time of Brillat-Savarin the connection between well-being and ingested food was clear, only in recent years have we discovered the mechanisms by which the gut senses food. With all its folds, villi, and microvilli, the gut is arguably the largest surface in the body. It is here where food is deconstructed into nutrients, ultimately giving rise to signals that control a range of bodily functions, including the desire to eat.
Because of the need to be aware of ingested food, the body has an intricate network of electrically excitable cells, carefully arranged into circuits and strategically distributed throughout the gut. These circuits convert food into electrical signals coordinating motility, secretion, food intake, and even mood and other behaviors. In the past, study of this network was limited to neurons of the enteric nervous system, but in recent years it has become clear that the neural network extends beyond enteric neurons. It is comprised of enteric glia, neurons of peripheral ganglia innervating the gut, intrinsic neurons, and specialized innervated epithelial sensors such as enteroendocrine cells. We believe it appropriate to call this network the “gut connectome.”
Two characters of the gut connectome, the glia and enteric neurons, arise from neural crest cells. They are immigrants to the bowel, traveling from the neural tube. Avetisyan et al. describe the molecular instruments orchestrating the migration of neural crest–derived cells to the intestine (2). Receptors, cofactors, and ligands meticulously coordinate the migration and transformation of progenitors into enteric ganglia. Ultimately, over 20 distinct types of neurons and accompanying glia are formed (3). These neurons are then guided by chemical cues to develop axons and establish synaptic connections with sensory and motor targets (4).
The gut connectome is a neural network built around food sensing. From the moment the fetus swallows amniotic fluid, the luminal contents of the digestive tract become a major factor in axonal pathfinding; after all, the network has to find the correct location to sense and utilize nutrients. Although there are reports of bacteria colonizing internal organs, including the gut, before birth (5), the gut microbiome, primarily after birth, serves as a beacon in the development of the network by priming the immune system and producing chemoattractants (6).
Kabouridis and Pachnis discuss how the gut microbiota increases the density of enteric nerves (7). The mechanisms appear to involve epithelial receptors, like those of the large family of toll-like receptors. In normal conditions, microbes in the gut do not have physical access to enteric nerves; therefore, their ability to alter the development and function of the enteric neural network is probably mediated by bacterial byproducts that sieve through the epithelium into the lamina propria or, more likely, through direct activation of epithelial sensory cells such as enteroendocrine cells. Enteroendocrine cells are in direct contact with the gut lumen and express molecular receptors specifically activated by bacterial ligands (8). If the integrity of the epithelial barrier is compromised by infection, the function of the neural circuitry is affected, as discussed by Mawe (9).
Enteroendocrine cells are essential for normal life. In their absence, severe diarrhea and early death occur (10). Like taste cells in the tongue or olfactory receptor cells in the nose, enteroendocrine cells are sensory epithelial cells. Recently their molecular sensing mechanisms have been uncovered, as reported by Psichas et al. (11). Enteroendocrine cells even express some of the same olfactory and taste receptors known to mediate the sense of smell and taste (12, 13). But unlike other epithelial sensors, they were thought to lack synaptic connections with nerves. Historically, enteroendocrine cells have been studied exclusively as a source of hormones. However, they have typical neural circuit features of sensory cells, including electrical excitability; functional voltage-gated channels; small, clear synaptic vesicles; nourishment from glial-derived neurotrophic factors; and a neuropod (14). It is through neuropods that enteroendocrine cells connect to nerves (15). This discovery opens up the possibility of the gut processing sensory signals in the lumen in a temporally precise, circuit-specific, and finely tuned manner.
Although enteroendocrine cells have a wide array of molecular receptors for chemicals in the lumen of the gut, the sensing of dietary fats has received much attention because of the obesity epidemic. The perception of fats differs between the mouth and small intestine. In the mouth, the taste of unsaturated fatty acids triggers signals to increase hunger, whereas in the small intestine, fatty acids trigger signals of satiety (16). The difference is in the epithelial sensory cells that transduce the chemical signal from a digested lipid into an electrical impulse in nerves. Some dietary fatty acids in the small intestine exert potent anorectic actions via a mechanism in which lipids bind and activate cell surface receptors such as GPR40, GPR41, or ILDR1 (17, 18). DiPatrizio and Piomelli discuss how endogenous lipids produced in the gut modulate appetitive behaviors (19). Sensory signals from fats and other nutrients are ultimately relayed via the vagus nerve to the brain, where fat ingestion is perceived.
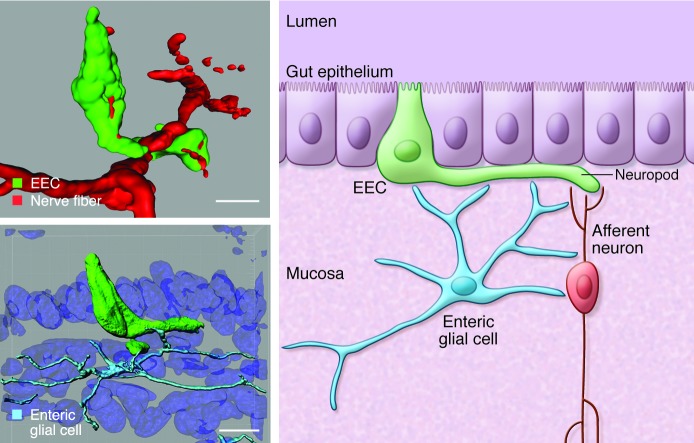
An important character of the gut connectome is the enteric glia. Discovered by Russian physiologist Dogiel in the late 1800s (20), enteric glia were first recognized as essential for normal gut function in 1998 (21). They are distributed throughout the mucosa and neuronal plexus of the gut and form a syncytium that is functionally coupled through gap junctions made of hemichannels such as connexin 43 (22). In this issue, Sharkey describes the role of enteric glia in barrier and defense functions of the gut and gastrointestinal motility disorders (23). Enteric glial cells also form neuro-glial junctions with neurons and, at least in the myenteric plexus, are known to receive cholinergic innervation (24). We have documented a physical association of enteric glia with enteroendocrine cells, highlighting how the enteric glial cell is a critical character in the gut connectome (Figure 1 and ref. 14).
Figure 1. The gut connectome: built for sensing food.
Top left: A sensory enteroendocrine cell (EEC) in the gut epithelium can be seen extending a neuropod to connect with an underlying nerve. Bottom left: Enteric glia underneath the epithelium extend processes to contact the neuropod of an enteroendocrine cell. Right: The innervation of enteroendocrine cells brings the possibility of afferent (gut-to-brain) signaling and possible efferent (brain-to-gut; not shown) signaling, which would allow the gut to compute sensory information from food to modulate whole-body metabolism and behaviors such as hunger and satiety. Figures adapted from PLoS One (14) and J Clin Invest (15).
Besides modulating the transmission of sensory information, enteric glia appear to be an important defense against pathogenic invasion. Pathogens like the JC virus and misfolded prion proteins are harbored by enteric glia. Both JC virus and prion proteins gain entry through the gut but affect the brain (25). This is an important observation because enteroendocrine cells are exposed to the gut lumen, where external viruses and prions first arrive. In an effort to unveil a gut-brain neural circuit, we recently used a modified rabies virus. This neurotropic virus infects enteroendocrine cells, and given the right conditions, the rabies virus spreads into the nervous system (15). This uncovered a conduit through which pathogens that are able to infect enteroendocrine cells could access first the peripheral then the central nervous systems. And, like astrocytes in the brain, enteric glia may also clear infections of the gut connectome.
The neuronal ensemble of sensory cells, neurons, and glia is modulated and shaped by the gut microbiome. Gut microbes can have mind-altering effects as discussed by Mayer et al. and, although the mechanisms remain unknown, it is clear that the ability of the gut connectome to process sensory information is involved (26).
Alterations in the gut connectome have also been observed in patients undergoing gastric bypass surgery. The procedure dates back to 1966, when Dr. Edward Mason first implemented it to treat obese patients (27). The procedure has since been refined, and there are several variations of it, the most popular being Roux-en-y (RY) gastric bypass. Manning et al. explain that RY gastric bypass forces food to bypass the stomach and enter directly into the distal small intestine (28). The effects on diabetes and body weight are remarkable. Three years after surgery almost seven of ten patients experience diabetes remission, and remarkably only six months after the surgery, an average patient loses over one-quarter of his initial body weight (29). The effects were thought to be due to the altered anatomy, but more recently it has become clear that neuroendocrine mechanisms are involved: in simple terms, the bodyweight set-point is reset.
Perhaps the most remarkable observation learned from RY gastric bypass surgery is the alteration of food perception. RY gastric bypass reduces the preference for sugars and even the cravings for sweets and fatty foods (30). Rewiring of the gut connectome indeed alters where nutrients are sensed, how nutrients are sensed, and our perception of food. Jean Brillat-Savarin was right after all; we truly are what we eat.
Acknowledgments
This work was supported in part by NIH grants DK091946, DK098796, and VA grant I01BX002230 (to R.A. Liddle) and by NIH grant DK103832 (to D.V. Bohórquez).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(3):888–890. doi:10.1172/JCI81121.
References
- 1. Brillat-Savarin JA. The physiology of taste: or meditations on transcendental gastronomy. Washington, DC, USA: The Heritage Press; 1949. [Google Scholar]
- 2.Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. 2015;125(3):899–907. doi: 10.1172/JCI76307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81(1–3):87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 4.Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305(1):G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 7.Kabouridis PS, Pachnis V. Emerging roles of gut microbiota and the immune system on the development of the enteric nervous system. J Clin Invest. 2015;125(3):956–964. doi: 10.1172/JCI76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogunovic M, et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1770–G1783. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125(3):949–955. doi: 10.1172/JCI76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellitzer G, et al. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120(5):1708–1721. doi: 10.1172/JCI40794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest. 2015;125(3):908–917. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang HJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132(5):1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9(2):e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125(2):782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra R, Wang Y, Shahid RA, Vigna SR, Freedman NJ, Liddle RA. Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion. J Clin Invest. 2013;123(8):3343–3352. doi: 10.1172/JCI68587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou AP, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140(3):903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiPatrizio NV, Piomelli D. Intestinal lipid–derived signals that sense dietary fat. J Clin Invest. 2015;125(3):891–898. doi: 10.1172/JCI76302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dogiel AS. Über den bau der ganglien in den geflechten des darmes und der gallenblase des menschen und der säugetiere . Arch Anat Physiol (Leipzig) Anat Abt Jg. 1899:130–158. [Google Scholar]
- 21.Bush TG, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93(2):189–201. doi: 10.1016/S0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 22.McClain JL, et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146(2):497–507 e1. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125(3):918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautron L, Rutkowski JM, Burton MD, Wei W, Wan Y, Elmquist JK. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J Comp Neurol. 2013;521(16):3741–3767. doi: 10.1002/cne.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selgrad M, et al. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut. 2009;58(1):25–32. doi: 10.1136/gut.2008.152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason EE, Ito C. Gastric bypass in obesity. Surg Clinics N Am. 1967;47:1345–1351. doi: 10.1016/s0039-6109(16)38384-0. [DOI] [PubMed] [Google Scholar]
- 28.Manning S, Pucci A, Batterham RL. Roux-en-Y gastric bypass: effects on feeding behavior and underlying mechanisms. J Clin Invest. 2015;125(3):939–948. doi: 10.1172/JCI76305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courcoulas AP, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring). 2014;22(5):E13–E20. doi: 10.1002/oby.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]