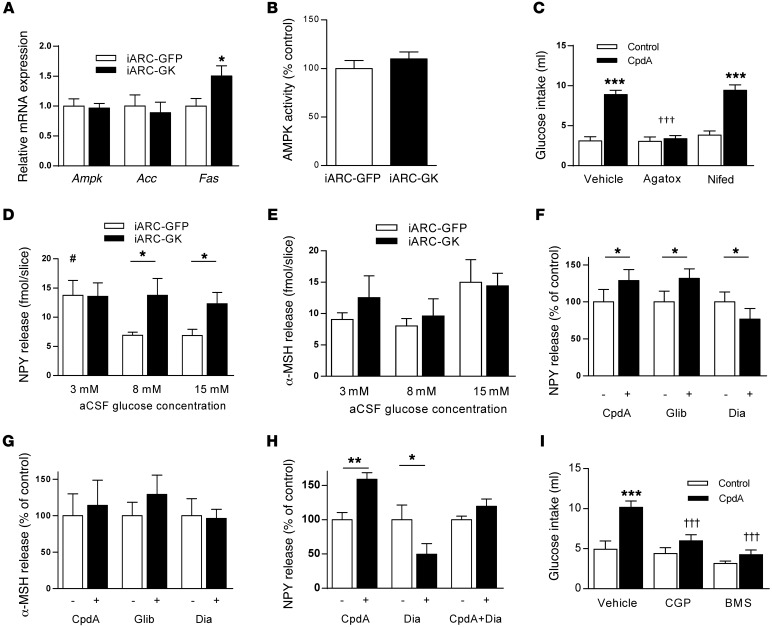
Figure 5. Effect of increased arcuate nucleus glucokinase on NPY and α-MSH and effect of NPY antagonist and calcium channel blockade on glucokinase activator–stimulated glucose intake.
(A) Hypothalamic Ampk, Acc, and Fas expression in iARC-GFP and iARC-GK rats (n = 9). (B) Hypothalamic AMPK activity in hypothalami from iARC-GFP and iARC-GK rats (n = 10). (C) 2% w/v glucose solution intake 1 hour after intra-arcuate injection of vehicle ω-agatoxin-IVA (Agatox) or nifedipine (Nifed) and subsequent injection of CpdA or control in rats (n = 15). (D) Glucose-induced NPY release from hypothalamic slices of iARC-GFP or iARC-GK rats (n = 7 iARC-GFP; n = 8 iARC-GK). (E) Glucose-induced α-MSH release from hypothalamic slices of iARC-GFP or iARC-GK rats (n = 7 iARC-GFP; n = 8 iARC-GK). (F) NPY release from rat hypothalamic slices, following treatment with CpdA, glibenclamide, or diazoxide (n = 10–12). (G) α-MSH release from rat hypothalamic slices, following treatment with CpdA, glibenclamide, or diazoxide (n = 6–9). (H) NPY release from rat hypothalamic slices, following treatment with CpdA or diazoxide alone and in combination (CpdA+Dia) (n = 10–12). (I) 2% w/v glucose solution intake 1 hour after intra-peritoneal injection of vehicle CGP-71683 (CGP) or BMS-193885 (BMS) and subsequent intra-arcuate injection of CpdA or control in rats (n = 14). Data are presented as mean ± SEM. *P < 0.05 versus corresponding control values; #P < 0.05 versus control values from 8 mM and 15 mM glucose; **P < 0.00001 versus corresponding CpdA-injected group; †††P < 0.0001 versus vehicle CpdA-injected group; ***P < 0.0001.