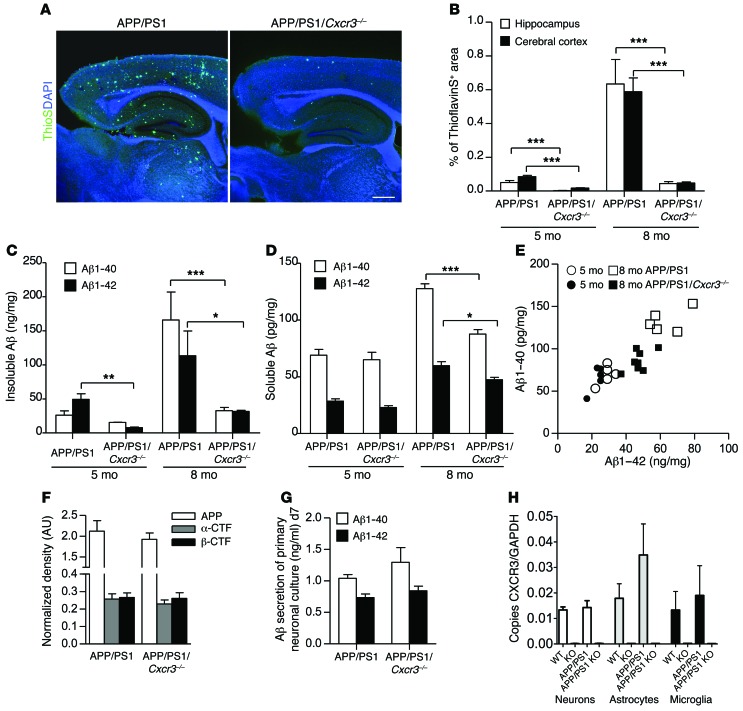
Figure 1. CXCR3 deficiency leads to a strong reduction of Aβ deposition in APP/PS1 mice.
(A) Brain sections of 8-month-old APP/PS1 and APP/PS1/Cxcr3–/– male mice were stained with ThioS to detect Aβ deposition. Scale bar: 500 μm. (B) Hippocampal and cortical regions were quantified. (C) ELISA measurement of Aβ1–40 and Aβ1–42 peptides documents a reduction in the insoluble brain fraction of APP/PS1/Cxcr3–/– mice. (D) At 5 months (5 mo), no significant changes in the composition of both soluble peptides were observed in APP/PS1/Cxcr3–/– compared with APP/PS1 mice. At 8 months, both Aβ peptides are significantly reduced in APP/PS1/Cxcr3–/– mice. (E) A scatter plot of soluble Aβ1–40/1–42 composition in 5- and 8-month-old APP/PS1 and APP/PS1/Cxcr3–/– mice is shown. Immunoblot analysis using a holo-APP antibody (CT15) indicates no effect of CXCR3 deficiency on APP processing in APP/PS1 mice at 5 months. (F) Densitometric analysis of holo-APP, α-CTFs, and β-CTFs after normalization to α-tubulin. (G) Unaltered Aβ1–40 and Aβ1–42 secretion of primary cortical APP/PS1/Cxcr3–/– neurons compared with the APP/PS1 genotype. n = 4 cultures of both genotypes. (H) Cxcr3 RNA detection by qPCR confirms the lack of CXCR3 in primary cultured neurons, astrocytes, and microglia. (A–F) Data are shown as mean ± SEM, n = 5–8 mice per group. (H) Data are shown as mean ± SEM, n = 3–5 primary cultures in each group. *P < 0.05; **P < 0.005; ***P < 0.001.