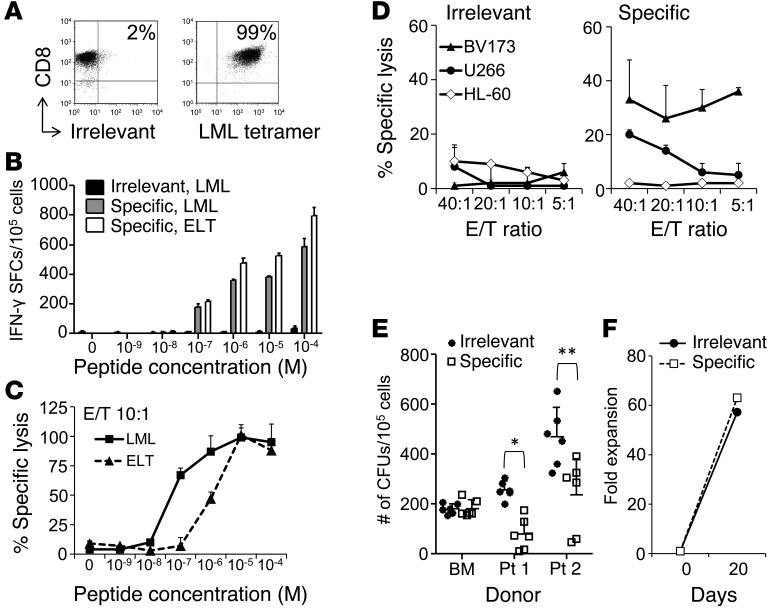
Figure 1. Survivin-specific T cell clone with antitumor effects in the absence of toxicity.
(A) FACS analysis of the survivin-specific T cell clone stained for CD8 and the LML-specific or irrelevant tetramer. (B) T cell avidity assessed by IFN-γ ELISpot assays of the irrelevant clone against the LML peptide (black bars) and the survivin-specific clone against the LML (gray bars) or the ELT peptides (white bars). SFCs per 105 cells. Data represent the mean ± SD of triplicate experiments. (C) T cell avidity assessed by 51Cr-release assay against LML- (squares, solid line) or ELT-pulsed T2 cells (triangles, dashed line). Data show the mean ± SD of triplicates of the specific lysis at a 10:1 E/T ratio. (D) Antitumor activity by 51Cr-release assay of an irrelevant (left panel) and a survivin-specific (right panel) clone derived from the same donor against the HLA-A*02+survivin+ target cell lines BV173 and U266 and the HLA-A*02–survivin+ target cell line HL-60. Data represent the mean ± SD of 3 technical replicates of 1 experiment. Two independent experiments were performed in triplicate. (E) Antileukemic activity and absence of toxicity to normal hematopoietic progenitor cells by CFU assay of the survivin-specific clone (white squares) and the irrelevant clone (black circles) against HLA-A*02+survivin+ primary leukemic blasts from 2 CML blast crisis patients and 1 HLA-A*02+ normal BM donor. Data represent the mean ± SD of 3 independent experiments performed in duplicate. *P < 0.001; **P = 0.001 by Student’s t test. (F) Absence of T cell fratricide, assessed as fold expansion over a 3-week culture period after superexpansion of the survivin-specific (white squares, dashed line) and irrelevant (black circles, solid line) clones.