Abstract
High-throughput sequencing of immunoglobulin repertoires in patients with multiple sclerosis reveals that antigen-experienced B cells traffic between the peripheral lymph nodes and the central nervous system, suggesting the involvement of peripheral immunity in the autoimmune pathogenesis of multiple sclerosis.
EMERGING ROLE FOR B CELLS IN MS
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS) that results from autoimmune-mediated destruction of the myelin sheath. Among the most prominent immunodiagnostic features of MS is the accumulation of B-cell secreted antibodies in the CNS, as evidenced by the presence of oligoclonal bands in the cerebrospinal fluid (CSF) and of ectopic lymphoid follicles enriched with B cells in CNS lesions 1. B-cell involvement is also supported by data showing that the number of B cells in CSF correlates positively with MS progression and that the CNS microenvironment in MS patients promotes B-cell persistence and expansion 2, 3. Moreover, several recent clinical findings demonstrate that B cell-targeted therapies profoundly mitigate disease activity in MS. Specifically, CD20 B-cell-depleting therapies significantly reduced the frequency of relapses and of new demyelinating lesions in phase II studies, and accumulating evidence suggests that immunotherapies that block immune-cell trafficking across the blood-brain barrier (BBB) reduce disease activity in part by impeding B-cell function 4.
While these features have been described, little is known about the mechanisms regulating the antigen-dependent selection and activation of B cells that populate the CNS. Further, the sites of B-cell affinity maturation that produce these putative pathogenic B cells are ill defined. In this issue of Science Translational Medicine, Palanichamy et al. and Stern et al., by deep sequencing immunoglobulin repertoires in MS, provide evidence of bi-directional migration of B cells between the CNS and periphery. Their complementary findings suggest that antigen-experienced B cells mature in peripheral lymph nodes before transmigrating to the CNS. These results provide insight into the trafficking of the presumed pathogenic B cells in MS and a framework by which peripheral deletion or modulation of specific B-cell subsets could provide therapeutic benefit.
CONNECTING PERIPHERAL BLOOD TO CSF B CELLS
In 2012, von Budingen and colleagues deep-sequenced the variable region genes of immunoglobulin heavy-chains (Ig-VH) in the CSF and in the peripheral blood of MS patients. They found that a population of B cells in the peripheral blood used the same immunoglobulin genes and complementarity-determining region 3 sequences as B cells in the CSF, providing evidence for the existence of bicompartmental lineages that connect peripheral B cells to CNS B cells in MS 5.
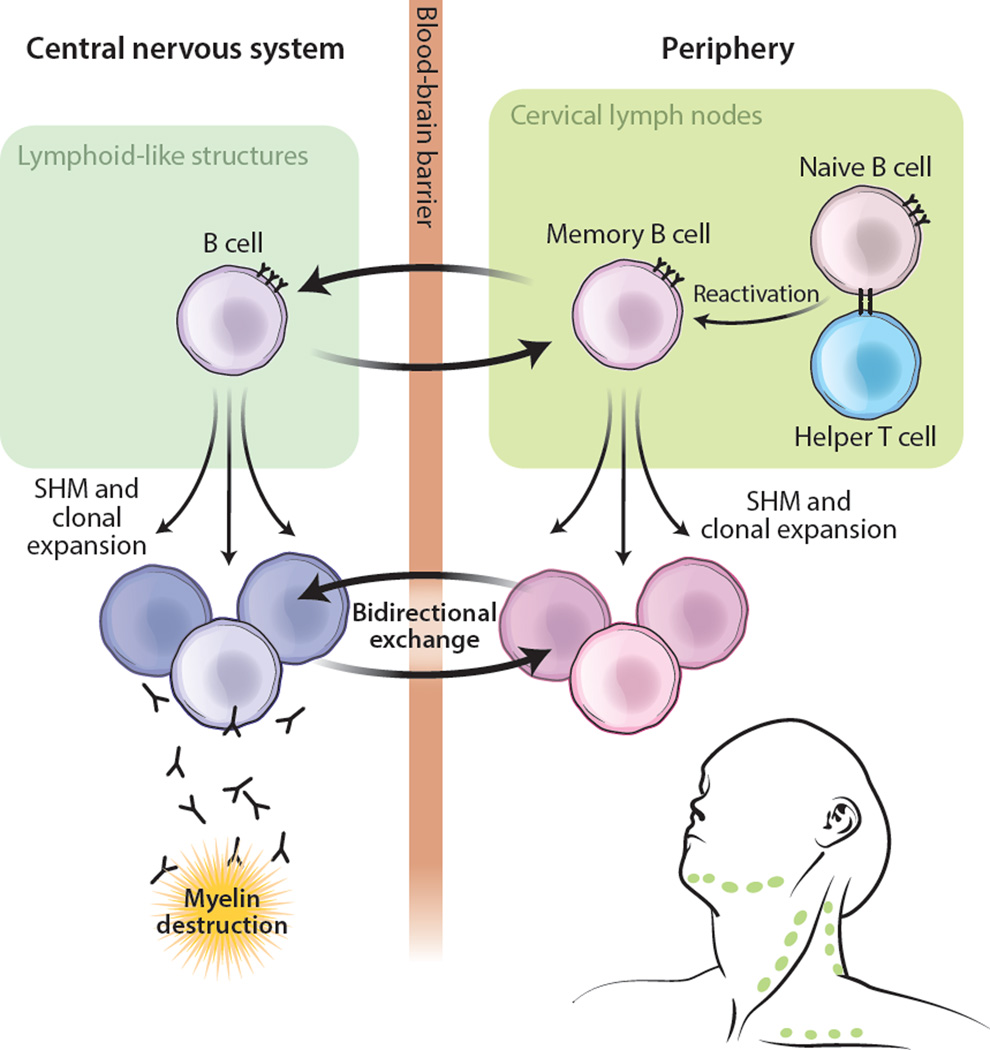
In this issue of Science Translational Medicine, Palanichamy and colleagues extend this line of investigation by delineating the functional subsets of peripheral B cells that share lineages with B cells in the CSF. By comparing the Ig-VH repertoire of cell-sorted peripheral B-cell subsets to that of B cells in patient-matched CSF, they find that peripheral class-switched memory B cells and plasma cells have the greatest degree of sequence overlap with CSF B cells, whereas peripheral unswitched memory B cells and naive B cells have the least. Thus it appears that it is primarily antigen-experienced B cells, and not naive B cells, that constitute the humoral immune axis spanning the peripheral blood and the CNS. Because B cells can differentiate into switched memory B cells and plasma cells only after a productive germinal-center reaction, this finding supports the emerging concept that MS pathology derives from successive rounds of CNS tissue damage, whereby early antigen encounters generate autoreactive memory B cells, and subsequent antigen encounters activate the memory B cells, resulting in B-cell mediated tissue destruction in the CNS (Fig. 1).
Figure 1. Peripheral antigen experience modulates CNS B-cell responses in MS.
Peripheral antigen exposure results in antigen priming of naive B cells and the formation of immunological memory. These B cells undergo affinity maturation, clonal expansion, and extravasation into the CNS. Formation of ectopic lymphoid-like structures in the CNS perpetuates the activation and affinity maturation of these B cells, which transmigrate between the CNS and periphery. B cells likely contribute to the pathogenesis of MS by producing autoantibodies, by serving as professional antigen-presenting cells, and/or by producing pro-inflammatory cytokines. SHM, somatic hypermutation.
DETECTING SITES OF B-CELL AFFINITY MATURATION
Where do these autoreactive B cells initially undergo affinity maturation? In order for the humoral immune system to develop affinity-matured antibody responses, B cells must traffic to germinal centers where activation signals trigger pro-survival signals, somatic hypermutation, and antibody class-switching. In this issue of Science Translational Medicine, Stern and colleagues compare the immunoglobulin repertoires in sectioned MS brain tissues to those in patient-matched cervical lymph nodes (CLN) draining the brain to determine whether B cells associated with MS gain antigen experience in peripheral lymphoid tissues or within the CNS.
They observe that B-cell populations containing sequences most closely resembling germline sequences—which they term "founder events"—are highly enriched in B-cell lineages that span both tissues despite their low frequency. This implies that B-cell lineages with members in both the CNS and CLN are prone to undergo additional rounds of affinity maturation.
The authors then examine lineage trees for the occurrence of founders within the CNS and CLN, and find that ~90% of founders are present in the CLN. Though it is impossible to definitively conclude where B cells first encounter antigen on the basis of sequence data alone, these results support a role for peripheral immunity in shaping the repertoire of CNS B cells, and thereby suggest that the initial antigen-dependent maturation of inter-compartmental B cells occurs in the periphery. This concept is supported by previous studies that identify neuronal-derived antigens in the CLN 6.
CLINICAL IMPLICATIONS AND OUTSTANDING QUESTIONS
The studies by Palanichamy et al. and Stern et al. highlight distinct but complementary intricacies of bi-directional B-cell exchange across the BBB in MS. Both studies conclude that bi-compartmental Ig-VH sequences derive from post-germinal-center B cells, as evidenced by class-switching to IgG and IgA and the accumulation of extensive somatic mutations. In some instances the sequences of bi-compartmental lineages that have a high number of mutations are present in the CNS tissues, and in other instances they are present in the periphery. This distribution of highly mutated sequences suggests a bidirectional exchange of B cells across the BBB and argues that affinity maturation can occur in both the CNS tertiary lymphoid structures and in the CLN (Fig. 1).
Targeted immunotherapies that inhibit B-cell trafficking might abrogate cross-compartmental B-cell trafficking and activation, and thereby provide benefit in MS. Additional studies are needed to determine how therapies such as Natalizumab and FTY720 affect B-cell trafficking and B-cell mediated inflammation. The bi-directional trafficking of B cells may also explain how therapies that deplete circulating B cells are able to reduce B cells in cerebral perivascular spaces 4, 7, which may be responsible for the therapies’ activity in MS. Although pathogenic B cells may exert their proinflammatory effects in the CNS, they appear to continually enter and exit the BBB, thereby enabling therapies such as Rituximab, Ocrelizumab, and Ofatumumab to reduce intrathecal B-cell numbers by targeting precursor and recirculating B cells in the periphery.
That it is the affinity-matured memory B cells that contribute to the immune continuum between the periphery and CNS has implications for the pathogenesis of MS. Because formation of memory B cells requires prior encounters with antigen and the successful completion of the germinal center reaction, these results suggest that prior inflammatory events that aberrantly expose myelin and neuronal antigens, or microbial antigenic mimics, to the immune system shape the autoimmune response in MS 3. Treatments that reduce the magnitude of inflammatory events in at-risk individuals or hamper the formation of immunological memory may avert the development of clinical MS.
These exciting findings, while offering a snapshot of antibody repertoires at a single time point, raise a number of questions about the role of B cells in MS. At what stage do potentially autoreactive B cells first encounter the antigens that initiate their selection and/or activation, and can this be linked to clinically distinct inflammatory phenotypes? In addition, most of the patients sequenced had relapsing-remitting MS or progressive MS, and thus had an advanced autoimmune response. Monitoring the antibody repertoires of individuals who are at-risk of developing MS may provide greater insights into the role of B cells in MS by showing how the B cell repertoire evolves over the course of the disease.
B cells are thought to contribute to MS plaque formation through the secretion of proinflammatory cytokines, the presentation of antigen to other immune cells, and/or antibody-mediated mechanisms 3, 4. While Palanichamy et al. and Stern et al. assume that B cells that transmigrate across the BBB contribute to MS pathology, it is impossible to determine whether these B cells are activated and homing to the CNS in response to one or more antigens without knowing what antigens these B cells target. Various papers have identified autoantigens present in MS patients 8, 9, but it is not known whether these antigens are targeted in all or only a subset of MS patients. Both papers report an overrepresentation of certain IGHV4 germline segments, a finding suggesting that selection of antibodies in MS is skewed toward particular antigen epitopes. Because MS is known to present with a diverse array of clinical features, improved classification of antibody repertoire heterogeneity and specificity has the potential to enable current and future treatments to more effectively be targeted to relevant patient populations.
Although the sequencing approaches utilized by Palanichamy et al. and Stern et al. do not provide pairing of heavy- and light-chain immunoglobulin genes expressed by individual B cells, high-throughput antibody repertoire sequencing approaches that enable such pairing are now available 10. Such methodologies will enable more comprehensive characterization of antibody repertoires in MS and other autoimmune diseases. Further, these next-generation technologies enable recombinant production of representative antibodies and thus identification of the antibodies’ autoantigen targets as well as assessment of their pathogenicity. Such characterization of representative antibodies will allow the “functional annotation” of antibody repertoires, and thereby link antibody repertoires to autoimmune specificity and disease pathogenesis.
Together, these studies reveal that MS is associated with an antigen-driven, affinity-matured humoral immune response in which antigen-activated B cells first mature in the CLN, then extravasate to the CNS, where they undergo further antigen-driven clonal expansion and continue to migrate back and forth across the BBB. These findings demonstrate how high-throughput sequencing of antibody repertoires can identify networks of lineage-related B cells and provide insights into B-cell pathobiology underlying MS.
REFERENCES AND NOTES
- 1.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuenz B, et al. Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PloS one. 2008;3:e2559. doi: 10.1371/journal.pone.0002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8:613–623. doi: 10.1038/nrneurol.2012.203. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann-Horn K, Kronsbein HC, Weber MS. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther Adv Neurol Disord. 2013;6:161–173. doi: 10.1177/1756285612474333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Budingen HC, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest. 2012;122:4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabriek BO, et al. In vivo detection of myelin proteins in cervical lymph nodes of MS patients using ultrasound-guided fine-needle aspiration cytology. J Neuroimmunol. 2005;161:190–194. doi: 10.1016/j.jneuroim.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Martin Mdel P, et al. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol. 2009;66:1016–1020. doi: 10.1001/archneurol.2009.157. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava R, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367:115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan YC, et al. Barcode-Enabled Sequencing of Plasmablast Antibody Repertoires in Rheumatoid Arthritis. Arthritis Rheumatol. 2014 doi: 10.1002/art.38754. [DOI] [PMC free article] [PubMed] [Google Scholar]