Abstract
Risk for depression is expressed across multiple levels of analysis. For example, parental depression and cognitive vulnerability are known markers of depression risk, but no study has examined their interactive effects on children’s cortisol reactivity, a likely mediator of early depression risk. We examined relations across these different levels of vulnerability using cross-sectional and longitudinal methods in two community samples of children. Children were assessed for cognitive vulnerability using self-reports (Study 1; n = 244) and tasks tapping memory and attentional bias (Study 2; n = 205), and their parents were assessed for depression history using structured clinical interviews. In both samples, children participated in standardized stress tasks and cortisol reactivity was assessed. Cross-sectionally and longitudinally, parental depression history and child cognitive vulnerability interacted to predict children’s cortisol reactivity; specifically, associations between parent depression and elevated child cortisol activity were found when children also showed elevated depressotypic attributions, as well as attentional and memory biases. Findings indicate that models of children’s emerging depression risk may benefit from the examination of the interactive effects of multiple sources of vulnerability across levels of analysis.
Keywords: depression, family history, childhood, cognitive vulnerability, cortisol
It is generally accepted that depression is an etiologically heterogeneous disorder that arises from interplay among multiple forms of risk across levels of analysis (e.g., Beck, 2008; Davey, Yucel, & Allen, 2008; Hankin, 2012). Consistent with this notion, multiple risk markers and vulnerability processes for the disorder have been identified. In particular, a family history of depression is one of the most robust markers of an individual’s own depression risk, with those with a first-degree relative with depression at approximately 3–10 times greater risk for developing depression (Goodwin & Jamison, 2007; Sullivan, Neale, & Kendler, 2000; Wallace, Schnieder, & McGuffin, 2002). The mechanisms by which this risk is transmitted are complex (Goodman & Gotlib, 1999), and likely include genetic influences (Levinson, 2009), poor parenting (Lovejoy, Graczyk, O’Hare, & Neuman, 2000; Wilson & Durbin, 2010), behavioral and learning processes (Ashman & Dawson, 2002), and exposure to adverse pre- and postnatal environments (Essex, Klein, Cho, & Kalin, 2002; Hammen, 1991), among other pathways.
However, it is important to note that familial depression is associated with an array of negative psychiatric outcomes in offspring other than depression (e.g., Barker, Copeland, Maughan, Jaffee, & Uher, 2012; Hammen, 2009), and that many with familial depression maintain adaptive psychological functioning despite this risk. Examining the risk associated with familial depression in conjunction with other established depression risks may lead to more refined models that account for why some children of depressed parents proceed to develop depression themselves as opposed to other disorders, such as externalizing psychopathology, and why some offspring do not develop significant depressive symptoms despite a positive family history of the disorder. Surprisingly, in light of its fundamental importance to developmental psychopathology perspectives (Cicchetti & Toth, 2009), very few studies have examined interactions between multiple markers of depression risk in predicting early vulnerability. The present study seeks to address this gap by examining whether cross-sectional and longitudinal associations between a parental history of depression and an important marker of offspring depression risk, heightened cortisol reactivity to stress, are moderated by children’s cognitive vulnerability to depression (CVD).
Cognitive theories of depression risk have generated a vast body of research over the past five decades, with work on this topic shifting in recent years toward the exploration of the utility of these models in adolescents and children (Abela & Hankin, 2008). Reviews of this literature support the claim that cognitive vulnerability in youth is an important prospective predictor of depressive symptoms (e.g., Hankin et al., 2009; Hankin, Snyder, & Gulley, 2013; Jacobs, Reinecke, Gollan, & Kand, 2008), usually when examined in conjunction with stressful life events. More specifically, most studies have focused on testing whether the interaction between cognitive vulnerability and stress predicts elevations in children and adolescents’ depressive symptoms, showing that stressful life events show stronger associations with depression when youth possess negative cognition, such as maladaptive attributional styles, information processing biases favoring enhanced processing of negative stimuli, and other aspects of depressive cognition (Abela & Hankin, 2008; Lakdawalla, Hankin, & Mermelstein, 2007).
However, other than stressful life events, the moderating effects of cognitive risk on other depression risk markers, such as family history of depression, have rarely been examined. This is a potentially important omission if multiple vulnerabilities have dynamic effects on risk, showing greater predictive power when considered in conjunction than individual risks examined in isolation. Interactions between cognitive risk and familial depression are to be expected, given that familial depression itself may be a marker of early childhood stress (Lovejoy et al., 2000; Wilson & Durbin, 2010; Hammen, 1991); if so, familial depression may simply represent an index of environmental stress that is moderated by children’s cognitive vulnerability to predict emerging depression and related outcomes. Conversely, familial depression is also frequently interpreted as a marker of genetic aspects of depression risk, given the evidence for the heritability of the disorder (e.g., Sullivan et al., 2000). If so, such genetic influences on biological processes, such as psychophysiological aspects of stress reactivity, might be potentiated by information processing biases that favor negative or threatening information, thus strengthening links between familial depression and depression-relevant outcomes. Despite these possible relationships, to our knowledge, no published research has examined whether these two established markers of depression risk interact.
Understanding the relationship between markers of depression risk and negative outcomes across time, prior to the age of risk for disorder, is crucial for developing comprehensive models of the developmental psychopathology of depression. Given that the condition is rare prior to adolescence (Garber, Gallerani, & Frankel, 2009), predicting depression per se in young children may be difficult due to its low prevalence; thus, research that predicts the emergence of other forms of risk may prove useful. With this in mind, we tested models predicting children’s hypothalamic-pituitary-adrenal (HPA) axis reactivity to social-evaluative stress, measured via cortisol, the glucocorticoid end product of the HPA system. Abnormal cortisol function and reactivity to stress are established markers of depression in adults and youth (Burke, Davis, Otte, & Mohr, 2005; Lopez-Duran, Kovacs, & George, 2009); further, cortisol reactivity appears to play a causal role in the development of the disorder (Azar, Paquette, Zoccolillo, Baltzer, & Tremblay, 2007; Feldman et al., 2009; Hasler, Drevets, Manji, & Charney, 2004; Oswald et al., 2006). In particular, it has been proposed that it may mediate associations between familial history and depression in offspring (Dougherty, Klein, Rose, & Laptook, 2011). If so, abnormal cortisol reactivity should emerge prior to significant depressive symptoms and thus potentially be more readily detectable in younger samples, making this a more tractable outcome variable for investigations of depression risk in childhood. Supporting this notion are numerous studies identifying deviant patterns of cortisol reactivity in the infant and child offspring of depressed mothers (Ashman, Dawson, Panagiotides, Yamada, & Wilkinson, 2002; Brennan, Pargas, Walker, Mewport, & Stowe, 2008; Diego et al., 2004; Essex et al., 2002). A small but supportive literature in adults also implicates cognitive factors, such as rumination and self-esteem, in cortisol function (Kuehner, Holzhauer, & Huffziger, 2007; Kuehner, Huffziger, & Liebsch, 2009; van Santen et al., 2011; Zoccola & Dickerson, 2012), although less attention has been directed toward identifying such associations in childhood. In fact, we know of no study investigating relationships between established indices of cognitive risk for depression and cortisol reactivity to stress in childhood, despite the fact that it has been posited that cognitive biases may confer risk for maladaptive HPA activation (Beck, 2008; Hankin, 2012). Overall, exploring interactions between theoretically relevant predictors of children’s cortisol reactivity to stress may help shed light on early processes that eventuate in depressive disorder in adolescence and adulthood.
We therefore examined whether two known markers of depression risk, a familial history of depression and cognitive vulnerability, predicted children’s cortisol reactivity to a standardized laboratory stressor. We used laboratory measures of attention and memory processing, as well as self-reported measures of cognitive risk, to assess multiple facets of cognitive vulnerability as a core risk to depression. Both laboratory measures of information processing (Garber et al., 2009; Hayden et al., 2013) along with self-report measures are useful in studies of CVD among youth. Alongside the finding that biased memory favoring negative information is perhaps the strongest cognitive marker of depression (Joormann, 2009; Matt, Vasquez, & Campbell, 1992), the use of information processing tasks in studies of CVD is strongly indicated as an adjunct to self-reported negative cognition. Furthermore, while ample previous work has found that children with a maternal history of depression are at heightened risk for the development of CVD (Garber & Flynn, 2001; Garber & Martin, 2002), associations between fathers’ depression history and child risk are poorly understood and understudied in the field, despite their implications for child outcomes (e.g., Connell & Goodman, 2002). We therefore expanded previous work by including measures of both maternal and paternal depression in one of our samples. We examined these questions in two studies, one of which used cross-sectional methods (Study 1), and next, in a longitudinal sample (Study 2) to test the hypothesis that the association between parental depression history and children’s cortisol reactivity to stress would be stronger among children with heightened cognitive vulnerability to depression.
Study 1
Participants
Children and adolescents were recruited by letters sent home to families with a child in 3rd, 6th, or 9th grades of public schools. Interested parents called the laboratory and responded to a brief phone screen that established that both the parent and child were fluent in English, and the child did not carry an autism spectrum or psychotic disorder and had an IQ > 70. Participants were 244 youth ranging in age from 9–15 (M=11.4, SD=2.27). The sample was approximately evenly divided by sex (boys: 44%, girls: 56%) and grade (32% 3rd grade, 32% 6th grade, 36% 9th grade). Ethnicity was as follows: Caucasian: 64%, African American: 8%, Latino: 7%, Asian/ Pacific Islander: 5%, Other/Mixed Race: 16%. Of the caretakers who participated in the study, 83% were mothers; the rest were fathers. Across all caretakers, 70% were married, 9% single, 20% divorced or separated, and 1% widowed. Median annual parental income was $75,000 (range $10,000 to $900,000), and 22% of the youth received free/reduced lunch at school.
The primary caretaker and the child participant visited the laboratory for the assessment. This consisted of a battery of questionnaires completed by youth and parents about the child, diagnostic interviewing, and collection of youth cortisol via saliva. Parents provided informed written consent for their own and their child’s participation; youth provided written assent. To allow for a decrease in any cortisol reactivity that may have occurred in response to arriving at the laboratory, the pair completed the stress paradigm after an hour in the laboratory. During this time, youth were asked to complete questionnaires and interviews. For 45 minutes after the challenge, youth completed additional questionnaires so that cortisol recovery could be assessed. On average, baseline cortisol samples were collected at 17:00 (range 16:00–18:30). Samples were then taken at 15-minute intervals until 60 minutes had passed since the onset of the stress paradigm. Diagnostic interviewing occurred in person during the laboratory visit, but after the laboratory stressor and saliva collection had been complete in case participants found this interview to be stressful.
Method
Depressive Symptoms
The Children’s Depression Inventory (CDI; Kovacs, 1985) was used to assess youths’ depressive symptoms. The CDI has good reliability and validity (Klein, Dougherty, & Olino, 2005). Internal consistency (α) was .89. The range of CDI scores from this sample (mean CDI was 6.25; SD = 5.4, range 0–35) was comparable to published norms (Kovacs, 1985) and prior research with community samples (Petersen et al., 1993).
Negative Cognitive Style
The Children’s Cognitive Style Questionnaire—Self implications subscale (CCSQ-S; Abela, 2001) was used to measure youths’ level of negative inferences about their self-concept based on the hopelessness theory of depression (Abramson, Metalsky, & Alloy, 1989). Youth are presented with hypothetical scenarios (e.g., “some kids that you know say they don’t like you”; “you get a bad grade in school”) in which they are asked to imagine negative events happening to them and then use a 3-point Likert scale to choose the response that best describes the way they would think (e.g., This does not make me feel bad about myself; This makes me feel a little bad about myself; and This makes me feel very bad about myself). The CCSQ-S has demonstrated good reliability and validity in prior research with children and adolescents (Abela, 2001). Internal consistency was .82.
Parental mood and anxiety disorder
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-NP; First et al., 1996) was used to assess caretakers’ lifetime history and current level of depressive and anxiety disorders at baseline. Diagnostic interviewers, who all had a minimum of a bachelors degree and most were advanced graduate students in clinical psychology, completed an intensive training program for assigning DSM-IV diagnoses. To assess interrater reliability, an independent trained rater who was blind to parent diagnostic status randomly evaluated 15% of the SCID interviews (N=37). Reliability was excellent (κ= .91), and presence/absence of diagnoses was confirmed. Considering the high level of familial aggregation between Major Depressive Disorder (MDD) and dysthymic disorder (DD; Klein et al., 1995; Klein, Shankman, Lewinsohn, Rhode, & Seeley, 2004), and the evidence that individuals with DD almost invariably develop MDD (Klein, Shankman, & Rose, 2006), MDD and DD were collapsed into a single category reflecting either depressive disorder. We also included cases of Minor Depressive Disorder (mDD), as these were associated with clinically significant impairment/distress. In our sample, half of the caretakers had a history of clinical depression (28.9% Definite MDD, 10.5% Probable MDD, 18.2% Definite mDD, and 5.2% DD; 53% of the primary caregivers who were mothers [N = 202] and 42 [37%] of the caregivers who were fathers). While these rates may appear high, considering only the rates of definite MDD and DD in the sample shows that these were consistent with those found in epidemiological studies (e.g., Kessler et al., 2005). Because depression is highly comorbid with anxiety disorders, and because anxiety disorders are also linked to cortisol reactivity (e.g., Yoon & Joormann, 2012), we included parental history of anxiety disorder in analyses; 31.3% of caretakers met DSM-IV criteria for a history of any DSM-IV anxiety disorder, except simple phobia, over the lifetime. Of those caregivers with a depressive disorder, 53% also met criteria for an anxiety disorder.
Stress paradigm and cortisol assessment
The stress paradigm (see Badanes, Watamura, & Hankin, 2011) included a 5–10 minute parent-child conflict discussion in which the parent and child were asked to talk about a recent fight or argument. Then, youth auditioned for a “reality TV show” by giving a speech directly into a video camera while their parent watched; youth were instructed that judges would evaluate their performance. Given the lack of consensus on stress/challenge paradigms that elicit cortisol reactivity across different ages (Gunnar, Talge, & Herrera, 2009), this laboratory challenge task was selected because it was expected to be developmentally appropriate across 3rd, 6th, and 9th graders, and it involved the essential elements (e.g., threat of social rejection and social evaluation, anticipatory and processive stress) known to activate the HPA axis in youths and adults (Lupien, McEwen, Gunnar, & Heim, 2009). Prior research (e.g., Badanes et al., 2001; Hankin, Badanes, Abela, & Watamura, 2010) using this stress elicitation paradigm has demonstrated its predictive validity for theoretically relevant outcomes.
Saliva samples were obtained via synthetic salivette collection devices (Sarstedt, Nuembrecht, Germany). Saliva was extracted by centrifuging for 4 minutes at 2500 RPM. Vials and salivettes were frozen at −20° C until data collection was complete. Samples were then defrosted and batched for assay in groups of 36 and were assigned to batches; all samples from the same child were analyzed in the same batch. Samples were sent to the Biochemical Laboratory, Psychobiology, University of Trier, Germany to be assayed. Cortisol levels were determined by employing a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA; Hoferl, Krist, & Buchbauer, 2005). For samples retained in the analyses described next, the mean interassay coefficients of variation (CV) for controls were 6.6% to 8.5%. For duplicates of the samples used in this study, the intraassay CV was 5%.
It is often the case that cortisol distributions are positively skewed (Gunnar & Talge, 2005), and this was true for the data obtained in this study. To address this, as is standard in this literature, a log10 transformation of the raw cortisol values produced unskewed cortisol values which were used in all analyses. As measures of cortisol reactivity to stress, we calculated the area under the curve (AUC) with respect to the ground (AUGg) and the increase (AUCi) in cortisol (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). For endocrinological data, AUCg is the total area under the curve of all measurements; it takes into account sensitivity and intensity differences in cortisol, whereas AUCi is calculated with respect to baseline cortisol levels and emphasizes change over time (Fekedulegn et al., 2007).
Results
Correlations among main variables for Study 1, as indicated in Table 1, show that negative cognitive style about the self was positively associated with children’s depressive symptoms and inversely related to AUCg. There were also some significant gender differences, such that girls reported higher levels of negative cognitive style and were more likely than boys to have a parent with a lifetime anxiety disorder. Also, older youth reported more depressive symptoms and higher AUCg.
Table 1.
Correlations between Study 1 variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. CCSQ | -- | .31** | −.05 | −.19 | .04 | .14* | .07 | .26** | .00 |
| 2. CDI | -- | .06 | −.10 | .03 | −.12 | −.18** | −.01 | .18** | |
| 3. MDD | -- | -- | .11 | .13 | .07 | .00 | −.03 | ||
| 4. PDD | -- | .49*** | .42*** | −.02 | .15 | .03 | |||
| 5.Parental AD | -- | −.09 | −.02 | .19* | .00 | ||||
| 6. AUCg | -- | .58*** | −.04 | .15* | |||||
| 7. AUCi | -- | −.03 | −.16* | ||||||
| 8. Child sex | -- | .06 | |||||||
| 9. Grade | -- | ||||||||
| Mean | 19.45 | 6.43 | 53% | 37% | 31% | −4.96 | −.86 | .-- | 6.01 |
| SD | 4.45 | 5.42 | -- | -- | -- | .92 | 1.25 | -- | 2.39 |
p < .01;
p < .05.
Note: CCSQ-S – Children’s Cognitive Style Questionnaire—Self Implications; CDI—Children’s Depression Inventory; MDD-maternal depressive disorder; PDD-paternal depressive disorder; Parental AD-parental history of any anxiety disorder; AUCg- Area under the curve-ground; AUCi- Area Under the curve-increase; Grade- child’s grade at baseline.
We next used multiple regression to test interactions between caregiver depression history and children’s cognitive risk in predicting indices of cortisol. In particular, we were interested in testing whether children’s cognitive risk moderated associations between caregiver depression history and offspring cortisol reactivity indexed via AUCg and AUCi. Children’s self-reported depressive symptoms and caregiver history of any anxiety disorder at baseline were treated as covariates. All variables were mean-centered as needed (Aiken & West, 1991).
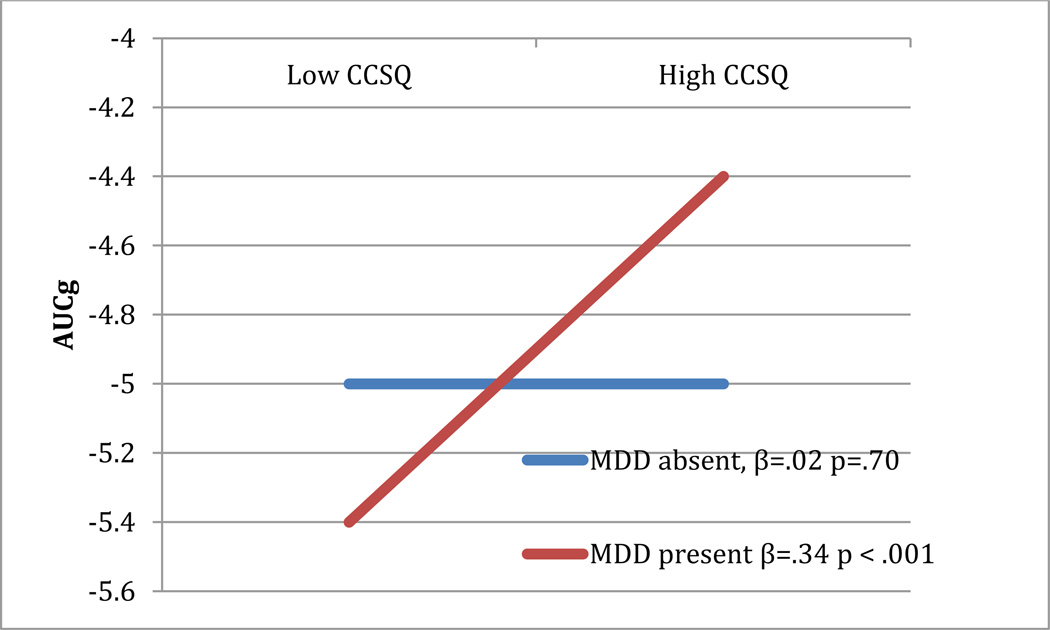
Results of this analysis, presented in Table 2 (top panel), show that caregiver history of mood disorder significantly interacted with youths’ negative cognitions about themselves in relation to youths’ cortisol reactivity. This significant interaction was decomposed by analyzing the strength of the relationship between youths’ negative cognitions about themselves and AUCg among those offspring of caregivers with and without a depression history. As hypothesized, there was a significant association between cognitive vulnerability and AUCg among offspring of caregivers with a depression history, whereas this relation was not significant among offspring whose caregivers did not have a depression history. Figure 1 graphically displays this interaction. The interaction between caregiver history of mood disorders and youths’ negative cognitive style did not predict AUCi.
Table 2.
Regression testing the interaction between parental depression history and child CCSQ-S predicting AUCg in Study 1.
| (mothers and fathers combined) | |||||||
|---|---|---|---|---|---|---|---|
| Overall Model | Change Statistics | ||||||
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 4, 239 | .05 | 2.68* | ||||
| Parental AD | −.01 | ||||||
| CDI | .01 | ||||||
| CCSQ-S | .20* | ||||||
| CDD | .10* | ||||||
| Step 2 | 5, 238 | .096 | 4.24*** | 1,238 | .045 | 9.98** | |
| Parental AD | .01 | ||||||
| CDI | .007 | ||||||
| CCSQ-S | .003 | ||||||
| CDD | 1.02*** | ||||||
| CCSQ-S X CDD | .97*** | ||||||
| (Fathers) | |||||||
| Overall Model | Change Statistics | ||||||
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 4, 38 | .21 | 1.79 | ||||
| Parental AD | −.18 | ||||||
| CDI | .001 | ||||||
| CCSQ-S | −.08 | ||||||
| PDD | −.35 | ||||||
| Step 2 | 5, 37 | .458 | 4.24*** | 1,37 | .248 | 11.87** | |
| Parental AD | .02 | ||||||
| CDI | .05 | ||||||
| CCSQ-S | .43* | ||||||
| PDD | 1.78*** | ||||||
| CCSQ-S X PDD | 2.28*** | ||||||
| (Mothers) | |||||||
| Overall Model | Change Statistics | ||||||
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 4, 198 | .07 | 2.87* | ||||
| Parental AD | .006 | ||||||
| CDI | .035 | ||||||
| CCSQ-S | .21* | ||||||
| MDD | .17* | ||||||
| Step 2 | 5, 197 | .11 | 4.24*** | 1,197 | .04 | 6.29* | |
| Parental AD | .025 | ||||||
| CDI | .03 | ||||||
| CCSQ-S | .03 | ||||||
| MDD | 1.03** | ||||||
| CCSQ-S X MDD | .90** | ||||||
p < .01;
p < .05;
p < .10.
Note: Parental AD-parental history of any anxiety disorder; CDI-Children’s Depression Inventory; CCSQ-S - Children’s Cognitive Style Questionnaire—Self Implications; CDD—Overall combined caregiver depressive disorder; MDD-maternal depressive disorder; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Figure 1.
Interaction between caregiver depression history and child CCSQ predicting AUCg
Note: CCSQ-Child Cognitive Style Questionnaire; MDD-Major Depressive Disorder for child’s caregiver (mother or father); AUCg-Area under the curve-ground.
In exploratory analyses examining the possibility that maternal or paternal depression history might be differentially important to the effect of caregiver in the model, we next separated caregivers into two groups based on whether they were mothers or fathers, and examined whether parental depression was differentially moderated by child cognitive vulnerability in predicting AUCg. Results are shown in Table 2. These show that the interaction was significant in the model with paternal depression; the analogous model with maternal depression was significant, but the effect was considerably smaller compared to that for fathers.
Study 2
Participants
A community sample of 205 seven-year-olds (96 boys; 46.83%) and their parents were recruited from Southwestern Ontario. Participants were recruited through a psychology department database, and advertisements placed in local newspapers and online. Children with a serious psychological or medical condition, as ascertained by a telephone screening process administered by a trained research assistant, were not eligible to participate. The mean age of children at baseline was 7.41 months (SD = .30). The Peabody Picture Vocabulary Test, Fourth Edition (PPVT-IV; Dunn & Dunn, 2007) was administered as a general measure of cognitive functioning. Children performed within the normal range (M = 111.92; SD = 12.15). Parents identified their child’s race as Caucasian (n = 180; 87.80%), Asian (n = 4; 1.95%) or other (n = 16; 7.80%). The vast majority of the children (n = 187; 91.22%) came from two-parent homes. Approximately half of the families participating (n = 103; 50.24%) reported a family income ranging from $40,000-$100,000 CAD; 26.83% (n = 55) of families reported a family income greater than $100,000, and 15.12% (n = 31) of families reported a family income of less than $40,000. Based on 2006 census data for this area (Statistics Canada, 2007), the sample was representative of the region from which it was recruited. Approximately two years after the collection of baseline data, a follow-up laboratory visit occurred when children were an average of 9.63 years old (SD = .38); 167 children (81% of the original sample) participated. Participants who completed follow-up procedures did not differ significantly on any study variables compared to those who completed the baseline assessment only (all ps > .09).
Method
Baseline assessment
Parent mood and anxiety disorder
The SCID was used to assess parents’ lifetime history of depressive disorder at baseline. In instances in which one of the biological parents was unavailable to complete a SCID (e.g., the participating parent did not know how to contact them), the Family History-Research Diagnostic Criteria (FH-RDC; Andreasen, Endicott, Spitzer, & Winokur, 1997) was used to obtain an assessment of history of psychopathology. Interviews, which were administered and scored by graduate students in clinical psychology who were blind to other study data, occurred during a home visit assessment that took place approximately 40 days (SD = 29.65) after children’s CVD was assessed as described in the next section.
We had clinical history information on 203 mothers and 196 fathers. The majority of mothers (n = 202; 99.51%) and most fathers (n = 183; 93.37%) completed the SCID (thus, clinical interview data was obtained via the FH-RDC for one mother and thirteen fathers). Inter-rater reliability was assessed by having each interviewer video-record a subset of interviews, which were then rescored by one of the other interviewers. Agreement between raters was high, with Cohen’s Kappa = 1.00, p < .001 for a diagnosis of any depressive disorder (N = 14). In our sample, 68 (33%) mothers and 34 (17%) fathers had a history of either MDD or DD; only 7 mothers and 7 fathers were currently experiencing an episode of depression or dysthymia. Interview data were not collected for minor depressive disorder in this study, in contrast to Study 1. In our sample, 70 (35%) mothers and 34 (17%) fathers had a history of a DSM-IV anxiety disorder. Agreement between raters was high, with Cohen’s Kappa = .83, p < .001 for a diagnosis of any anxiety disorder (N = 14).
Child cognitive vulnerability
Two aspects of CVD were assessed in Study 2: Self Referent Encoding Task (SRET) performance and attention bias to emotion. In order to produce mild negative affect and activate latent cognitive vulnerability (Taylor & Ingram, 1999; Teasdale & Dent, 1987), a mood induction procedure (MIP) was administered prior to the SRET, in which children were shown a sad movie clip (e.g., My Girl, Zieff, 1991; Stepmom, Columbus, 1998). Film clips have been shown to be successful in producing sad affect in children (e.g., Brenner, 2000), and the specific clips and procedures used here have been shown in two independent samples to increase children’s facial expressions of negative affect when coded by independent raters (Hayden, Klein, Durbin, & Olino, 2006), and to lead to decreases in children’s self-reported mood quality (Hayden et al., 2013).
The SRET (Kuiper & Derry, 1982) is a widely used information processing task used to assess memory biases for positive and negative self-referent information, as well as the extent to which individuals hold positive and negative self-views. In this task, participants are presented with a series of positive and negative adjectives and are asked to indicate whether each adjective is self-descriptive. This is followed by an unexpected free recall period in which participants are asked to recall as many of the presented adjectives as possible. Immediately following the MIP, children were presented with 26 words (13 positive and 13 negative) taken from previous research using this task with young children (Hayden et al., 2006). Words were presented on flash cards and spoken aloud by the experimenter. Following each word, children were asked “Is this like you?” Words were presented in a different random order for each participant with two neutral buffer words presented at both the beginning and the end of the list to address primacy and recency effects. This portion of the task was followed by an unexpected incidental recall period in which children were asked to recall as many of the words as possible from the list. As is typically done in research using this task (Gencoz, Voels, Gencoz, Pettit, & Joiner, 2000; Hammen & Zupan, 1984; Johnson, Joorman, & Gotlib, 2007; Taylor & Ingram, 1999), two indices of memory processing relevant to depression were calculated: a positive schematic processing score (the proportion of positive words rated as self-descriptive and recalled relative to all words rated as self-descriptive) and a negative schematic processing score (derived in the same manner using negative words).
The dot-probe task is a widely used computerized measure of information processing, designed to identify attentional biases in the processing of emotional information (e.g., MacLeod, Mathews, & Tata, 1986). In this task, participants are presented with a series of picture pairs typically consisting of one emotionally neutral image and one emotionally valenced image. At stimulus offset, one of the images is replaced with a probe stimulus and participants are required to identify, as quickly as possible, the location of the probe. Quicker responses to identify the location of probes replacing emotional images than probes replacing neutral images are thought to reflect attentional biases for information of this emotional valence (MacLeod et al., 1986). Children completed a computerized version of this task programmed using E-Prime software (Schneider, Eschman, & Zuccolotto, 2002). Four types of picture pairs (neutral-neutral, positive-neutral, sad-neutral, and threat-neutral) were created using images taken from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1997). The IAPS provides normative data for both the valence (i.e., pleasant versus unpleasant) and intensity of emotional arousal evoked by each image. Only stimuli for which norms existed for children this age were used. Pleasant images rated as low to moderate in arousal were chosen as positive stimuli. Sad and threat stimuli were similar in that they were both rated as unpleasant, but differed with respect to ratings of arousal. Specifically, low arousal-unpleasant images were chosen for sad stimuli and high arousal-unpleasant stimuli were chosen for threat stimuli. To reduce the number of analyses conducted, and as most research on depression risk has focused on reactivity to threat-related stimuli (e.g., Peckham, McHugh, & Otto, 2010), we used the dot-probe index capturing reactivity to threat in this study.
On each trial, picture pairs were presented on the left- and right-hand sides of the screen against a black backdrop for 1200 ms, after which both images disappeared and one was replaced with a probe stimulus (a small white dot). Children indicated the location of the probe via button press. The task consisted of three blocks of 60 trails (15 of each pairing type). New picture pairings were created for each of the three blocks so that no two images were paired on more than one trial. The probe location (left or right) was counterbalanced, and half of the neutral-valence trials were congruent (i.e., the probe was located at the same location as the emotional image) and half were incongruent (i.e., the probe was located at the same location as the neutral image). Trials within each block were presented in a different random order for each participant. Blocks were separated by a one-minute break. Consistent with established procedures in studies using the dot-probe paradigm with children (e.g., Dalgleish et al., 2003; Hankin et al., 2010; Heim-Dreger, Kohlmann, Eschenbeck, & Burkhardt, 2006; Hunt, Keogh, & French, 2007; Joormann, Talbot, & Gotlib, 2007; Kimonis, Frick, Fazekas, & Loney, 2006), data were cleaned by removing RT data for trials in which children committed errors (2.89% of trials) and for correct trials in which children responded faster than 100 ms or slower than 2000 ms (1.27% of correct trials).
Traditional bias scores (MacLeod & Mathews, 1988) were calculated, reflecting response time differences between congruent and incongruent trials, controlling for probe location (e.g., Threat Bias Score = 1/2 [(TrPl – TlPl) + (TlPr – TrPr)], where TrPl refers to the response time on trials in which the threat-related stimulus is located on the right and the subsequent probe is located on the left, etc). Using this method, positive scores reflect attentional biases towards threat, while negative scores reflect attentional biases away from threat.
Child depressive symptoms
The Depression Self-Rating Scale (DSRS; Birleson, 1981) is a 24-item self-report measure of depression in children, with items tapping affective, cognitive, behavioral, and somatic symptomatology (Asarnow & Carlson, 1985). The DSRS was read aloud to children by a research assistant at baseline assessment to address potential issues with reading comprehension. The DSRS demonstrates good psychometric properties (e.g., Asarnow & Carlson, 1985) and scores are related to symptoms of depression as assessed by other measures (e.g., Asarnow & Carlson, 1985; Birleson, 1981; Ivarsson, Gillberg, Arvidsson, & Broberg, 2002; Kashani, Reid, & Rosenberg, 1989). DSRS scores demonstrated good internal consistency (Cronbach’s α = 0.73). The average score in the current sample was 12.45 (SD = 5.30), which is comparable to that observed in other nonclinical samples (e.g., Asarnow & Carlson, 1985; Hayden et al., 2006).
Follow-up assessment
Trier Social Stress Test for Children (TSST-C)
Children participated in a modified version of the TSST-C (Buske-Kirschbaum et al., 1997). Upon arrival at the laboratory, children engaged in quiet activities (e.g., coloring, reading; watching a family-friendly movie) for 30 minutes to allow any potential increase in cortisol due to the arrival at the laboratory to return to baseline levels before sampling began (Tottenham, Parker, & Liu, 2001). Children were encouraged to stay seated and engage in minimal activity to avoid a cortisol increase related to physical activity (Jansen et al., 1999). After 30 minutes, a baseline salivary cortisol sample was collected, followed by the TSST-C. All visits began between 12:00pm and 3:30pm in the afternoon to minimize diurnal variation in cortisol levels.
After collecting the baseline sample, the child was brought to the testing room where they were told that they were being asked to complete a story for two “story judges,” actually two student research assistants. The main experimenter provided the beginning of the story to the child who was told that s/he would have 3 minutes to prepare a middle section and ending for the story. To increase the extent to which the task elicited anxiety, each child was told that his or her story should be as exciting as possible, and better than the stories of other children in the study. After the preparation period, the two research assistants entered the room. To increase the anxiety-provoking nature of the task, children were given a microphone to speak into and a video camera was held by one of the research assistants.
A research assistant directed the child through the TSST-C, prompting him or her to begin the story and to continue as necessary for a total duration of 5 minutes. After this, the research assistant instructed the child to complete a subtraction task by counting backwards from the number 758 by the number 7 as fast and as accurately as possible. The research assistant stopped the child and asked him/her to start again following all mistakes. The subtraction task also lasted 5 minutes. Following this, the child was asked to tell the research assistants about themselves and their personality in response to a series of prompts from the RA. Children were prompted to continue for 5 minutes or until all of the prompts had been repeated twice. Finally, the child was praised, thanked for participating, and given a prize by the research assistants.
Cortisol Sampling Procedure
In addition to the baseline sample previously described, cortisol samples were obtained at ten-minute intervals following completion of the task (i.e., at 0, 10, 20, and 30 minutes following the end of TSST-C) for a total of four samples post-stressor. Three children provided a baseline sample only, as their participation in the TSST was ended early due to the children becoming distressed; these participants are excluded from analyses. Cortisol can be readily indexed noninvasively through salivary assays, and such methods have been found to yield cortisol levels comparable to serum cortisol levels collected from blood samples (Dorn, Lucke, Loucks, & Berga, 2007); hence, this approach is more feasible and appropriate in research aimed at characterizing stress responsivity in childhood (Kryski, Smith, Sheikh, Singh, & Hayden, 2011). To collect saliva, the children were asked to chew on an absorbent cotton dental roll until it was wet with saliva; saliva was subsequently expunged from the rolls for analysis. All samples were frozen immediately following the laboratory visit. Samples were later taken to a laboratory at the University of Western Ontario to be assayed in duplicate using an expanded range, high sensitivity, salivary cortisol enzyme immunoassay kit (Salimetrics, State College, PA). The mean interassay coefficients of variation (CV) for controls were 4.1% to 6.8%. For duplicates of the samples used in this study, the intraassay CV was 3.6%. It is often the case that cortisol distributions are positively skewed (Gunnar & Talge, 2005) and this was true for the data obtained in this study. To address this, as is standard in this literature, a log10 transformation of the raw cortisol values produced unskewed cortisol values, which were used in all analyses. As in Study 1, we calculated the area under the curve (AUC) with respect to the ground (AUGg) and the increase (AUCi) in cortisol (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003).
Results
Correlations between all major study variables are shown in Table 3. Measures of children’s cognitive vulnerability at age 7 tended to be only modestly inter-correlated. A lifetime history of depressive disorder in mothers was unrelated to children’s CVD and cortisol indices at age 9; however, paternal depression was associated with SRET negative processing scores, threat bias scores from the dot-probe task, and AUCg, indicating that children with a paternal history of depression had more negative memory biases for self-referent information, a greater attentional bias for threat-related information, and excreted more cortisol over the course of the TSST at age 9. The parental anxiety disorder composite was positively related to both parents’ depression histories as well as children’s AUCg scores. Child age at baseline and sex were not significantly related to any other study variables. Child depressive symptoms were negatively related to SRET positive processing scores, positively related to threat bias scores on the dot probe task, and positively related to SRET negative processing scores (at trend level).
Table 3.
Correlations between Study 2 variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SRET-P | -- | −.15* | −.05 | .00 | .04 | .05 | .09 | .08 | .09 | .11 | −.15* |
| 2. SRET-N | -- | −.12 | −.07 | .16* | −.01 | −.07 | −.08 | −.11 | .03 | .12† | |
| 3. DP-TB | -- | .00 | .16* | −.05 | −.07 | −.11 | .06 | −.02 | .20** | ||
| 4. MDD | -- | .14† | .21** | .01 | .10 | .05 | −.10 | .09 | |||
| 5. PDD | -- | .17* | .19* | −.05 | −.01 | −.08 | .09 | ||||
| 6. Parental AD | -- | .16* | .05 | −.11 | −.01 | .06 | |||||
| 7. AUCg | -- | .34** | −.12 | .00 | −.12 | ||||||
| 8. AUCi | -- | −.07 | .09 | −.15† | |||||||
| 9. Child sex | -- | .02 | −.11 | ||||||||
| 10. Age | -- | .00 | |||||||||
| 11. DSRS | -- | ||||||||||
| Mean | .18 | .02 | 16.36 | -- | -- | -- | −4.55 | .52 | -- | 7.41 | 12.45 |
| SD | .13 | .03 | 70.09 | -- | -- | -- | 1.21 | 1.09 | -- | .30 | 5.30 |
p < .01;
p < .05;
p < .10.
Note: SRET-P – Self-referent encoding task positive processing; SRET-N- Self-referent encoding task negative processing; DP-TB-dot-probe threat bias; MDD-maternal depressive disorder; PDD-paternal depressive disorder; Parental AD-parental history of any anxiety disorder; AUCg- Area under the curve-ground; AUCi- Area under the curve-increase; Age- child age at baseline; DSRS-Depression Self-Rating Scale.
We next used multiple regression to examine interactions between maternal and paternal depression history and children’s cognitive risk at age 7 in predicting indices of cortisol reactivity at age 9. In particular, we were interested in testing whether children’s cognitive risk moderated associations between parental depression and offspring cortisol reactivity indexed via AUCg and AUCi. Children’s self-reported depressive symptoms at baseline were treated as a covariate as was parental history of any anxiety disorder (to reduce the number of model terms, this variable was a composite reflecting whether either parent had ever met criteria for an anxiety disorder; results were virtually identical when maternal and paternal anxiety history were included as separate variables). Initial models examined interactions between maternal and paternal depression with each measure of child cognitive risk predicting AUCg and AUCi; when an interaction term was not significant, it was dropped from the final model to increase model parsimony and statistical power. All variables were mean-centered as needed (Aiken & West, 1991).
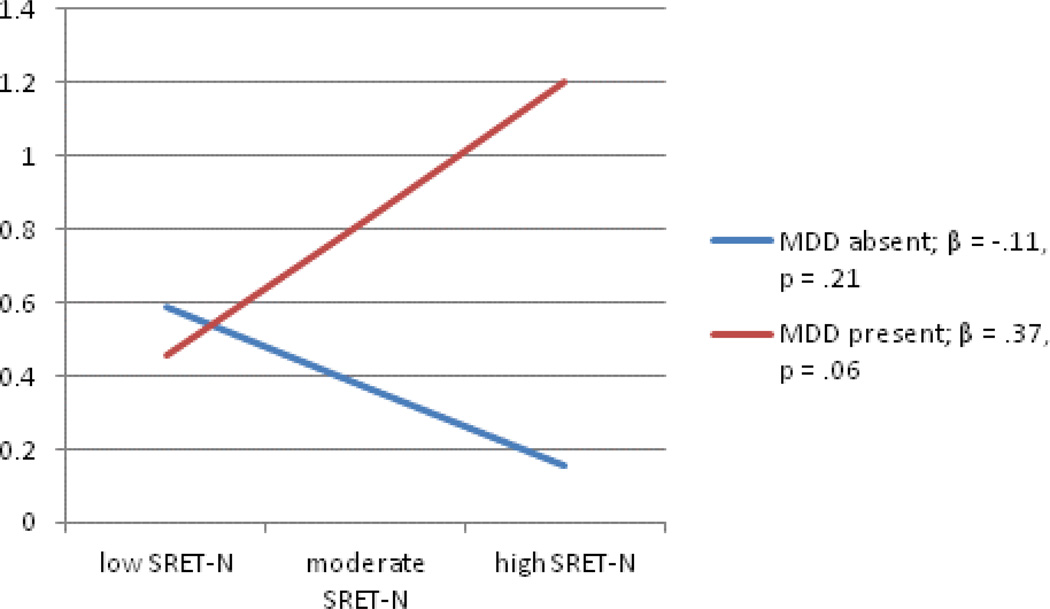
As we had four measures of cognitive risk and two indices of cortisol activity, we ran eight models in total for each measure of cognitive risk, testing whether it moderated associations between maternal and paternal depression and AUCg and AUCi. To conserve space, models with nonsignificant interactions are not presented here; these include three of the four models predicting AUCi. The regression testing the interaction between maternal depression history and child SRET-N predicting AUCi did yield a significant interaction term (Table 4), by which the significant main effect of maternal depression history on children’s cortisol indexed by AUCi was qualified by its interaction with SRET negative processing. Figure 2 depicts this interaction, which shows the significant, positive association between SRET negative processing scores and children’s AUCi in the context of a positive maternal depression history; the small, negative association between these scores and AUCi was not significant for children whose mothers had no history of depression.
Table 4.
Regression testing the interaction between maternal depression history and child SRET-N predicting AUCi
| Overall Model | Change Statistics | ||||||
|---|---|---|---|---|---|---|---|
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 5, 152 | .04 | 1.28 | ||||
| Parental AD | .02 | ||||||
| DSRS | −.15† | ||||||
| SRET-N | −.03 | ||||||
| MDD | .13 | ||||||
| PDD | −.05 | ||||||
| Step 2 | 6, 151 | .07 | 1.94† | 1,151 | .03 | 5.09* | |
| Parental AD | .04 | ||||||
| DSRS | −.14† | ||||||
| SRET-N | −.11 | ||||||
| MDD | .17* | ||||||
| PDD | −.03 | ||||||
| SRET-N X MDD | .21* | ||||||
p < .01;
p < .05;
p < .10.
Note: Parental AD-parental history of any anxiety disorder; DSRS-Depression Self-Rating Scale; SRET-N- Self-referent encoding task negative processing; MDD-maternal depressive disorder; PDD-paternal depressive disorder; AUCi-Area under the curve-increase.
Figure 2.
Interaction between maternal depression history and child SRET-N predicting AUCi
Note: MDD-maternal depressive disorder; AUCi-Area under the curve-increase; SRET-N- Self-referent encoding task negative processing.
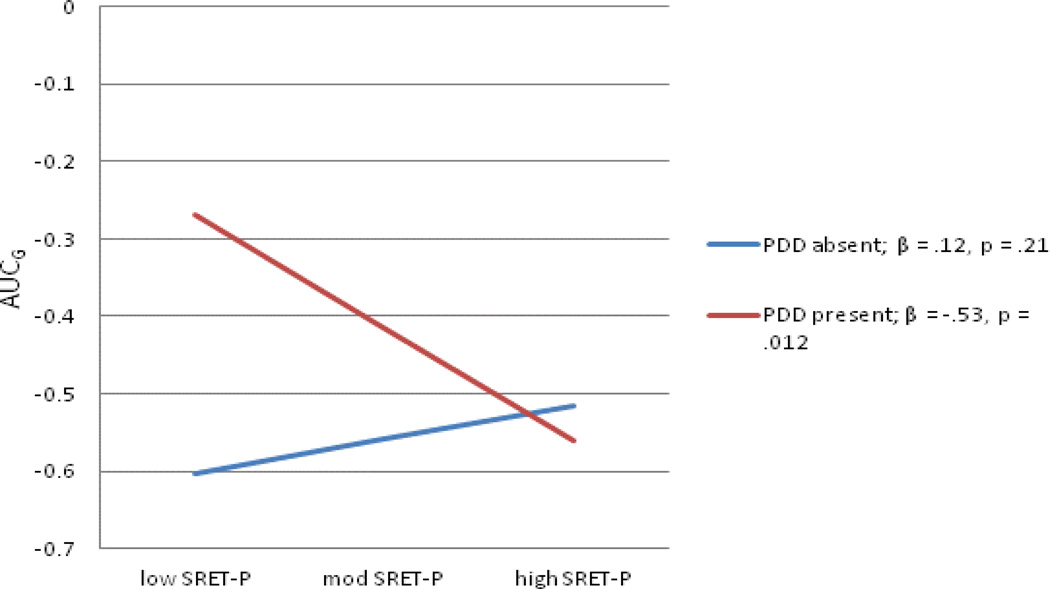
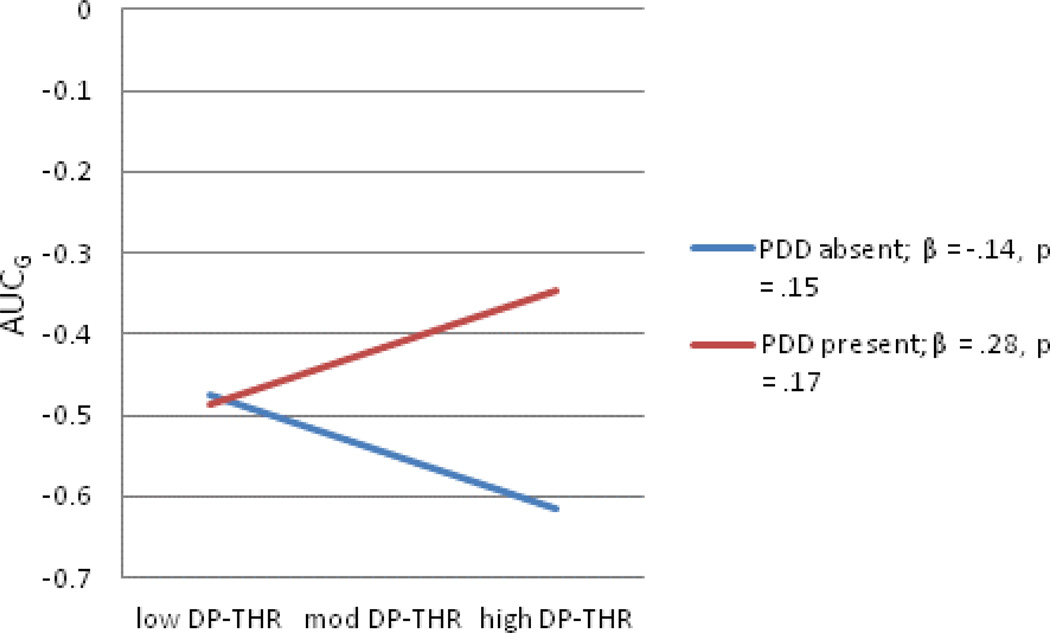
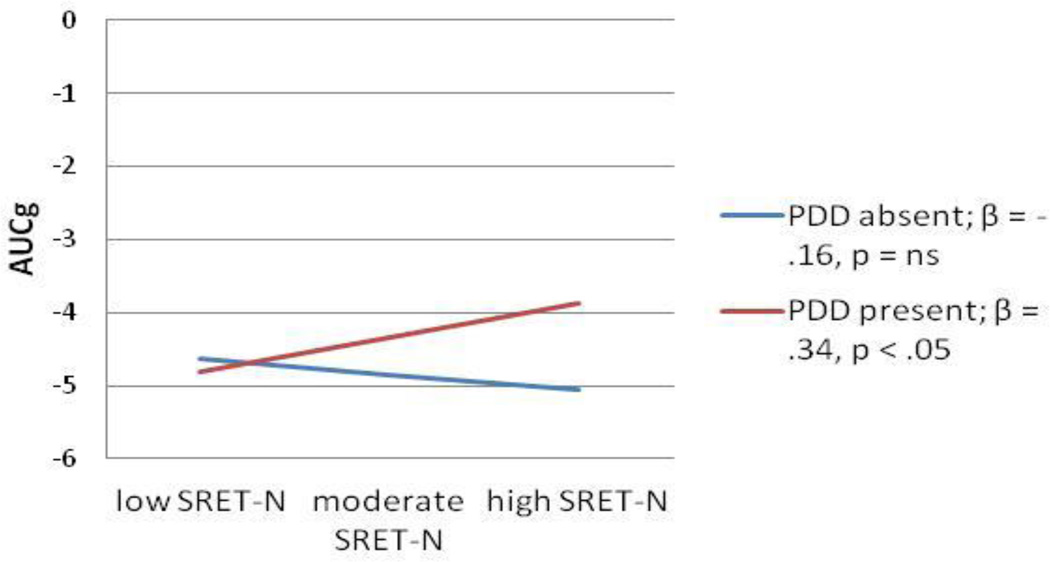
Regarding interactions between children’s cognitive risk and parental depression predicting AUCg, two of the four interaction terms tested were significant, both with respect to the moderation of paternal depression history by indices of children’s cognitive risk. An additional interaction between paternal depression and children’s dot-probe threat bias scores was a trend in predicting AUCg scores; because of the novelty of our study and analyses, we explore this trend here, acknowledging the importance of future attempts at replication. Full models are shown in Tables 5–7 and interactions depicted in Figures 3–5. As the pattern of effects was quite consistent across these three analyses, they will be discussed as a group. In each case, the association between cognitive risk and AUCg scores was not significant for children without a paternal history of depression. For SRET positive and negative information processing, greater cognitive risk was significantly related to greater cortisol production when children’s fathers’ had a history of depression; although the pattern was the same for threat bias indexed using the dot probe task, the slope did not reach significance.
Table 5.
Regression testing the interaction between paternal depression history and child SRET-P predicting AUCg
| Overall Model | Change Statistics | ||||||
|---|---|---|---|---|---|---|---|
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 5, 152 | .08 | 2.49* | ||||
| Parental AD | .14† | ||||||
| DSRS | −.13 | ||||||
| SRET-P | .06 | ||||||
| MDD | −.01 | ||||||
| PDD | .19* | ||||||
| Step 2 | 6, 151 | .13 | 3.77** | 1,151 | .06 | 9.46** | |
| Parental AD | .13 | ||||||
| DSRS | −.14† | ||||||
| SRET-P | .16† | ||||||
| MDD | .06 | ||||||
| PDD | .19* | ||||||
| SRET-P X PDD | −.26** | ||||||
p < .01;
p < .05;
p < .10.
Note: Parental AD-parental history of any anxiety disorder; DSRS-Depression Self-Rating Scale; SRET-P – Self-referent encoding task positive processing; MDD-maternal depressive disorder; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Table 7.
Regression testing the interaction between paternal depression history and child DP-TB predicting AUCg
| Overall Model | Change Statistics | ||||||
|---|---|---|---|---|---|---|---|
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 5, 150 | .08 | 2.69* | ||||
| Parental AD | .13 | ||||||
| DSRS | −.12 | ||||||
| DP-TB | −.09 | ||||||
| MDD | −.01 | ||||||
| PDD | .21** | ||||||
| Step 2 | 6, 149 | .10 | 2.86* | 1,149 | .02 | 3.49† | |
| Parental AD | .12 | ||||||
| DSRS | −.12 | ||||||
| DP-TB | −.15† | ||||||
| MDD | −.02 | ||||||
| PDD | .17* | ||||||
| DP-TB X PDD | .16† | ||||||
p < .01;
p < .05;
p < .10.
Note: Parental AD-parental history of any anxiety disorder; DSRS-Depression Self-Rating Scale; DP-TB-dot-probe threat bias; MDD-maternal depressive disorder; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Figure 3.
Interaction between paternal depression history and child SRET-P predicting AUCg
Note: SRET-P – Self-referent encoding task positive processing; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Figure 5.
The interaction between paternal depression history and child DP-TB predicting AUCg
Note: DP-THR-dot-probe threat bias; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Discussion
Very little is known about the interplay across multiple levels of analysis among established markers of depression risk in predicting emerging vulnerability to depressive disorders. We examined whether associations between parental depression and children’s cortisol reactivity were moderated by another well-known marker of depression risk, children’s cognitive vulnerability to depression. Findings generally supported the notion that parental depression and cortisol reactivity are more strongly associated in the context of elevated levels of child cognitive vulnerability; indeed, a fairly consistent pattern emerged across two independent samples and multiple measures of cognitive risk for depression.
We were somewhat surprised to find greater evidence for main effects and interactions involving fathers’ depression, rather than mothers’ depression, in shaping children’s cortisol reactivity. The finding of stronger effects observed for fathers’ depression, relative to mothers, was obtained in both studies, strengthening the confidence in this unanticipated pattern. In light of the scarcity of research on the role of fathers’ depression in children’s cortisol reactivity to stress, it is difficult to relate our work to a larger, relevant literature. To our knowledge, only one study has examined the role of paternal depression in child HPA axis function; in this study, Laurent and colleagues (2013) found that paternal depressive symptoms interacted with maternal depressive symptoms to predict lower daytime cortisol in adopted offspring. Considered as a whole, these findings suggest that the impact of paternal depression on aspects of children’s cortisol function may be important, and that future studies of this vulnerability pathway should include assessments of fathers, as well as measures of mediators of this risk (e.g., paternal caregiving; paternal genetic risk for depression). The lack of knowledge on this topic is an important omission in the literature, given that research indicates that paternal depression is associated with youth psychopathology (Brennan et al., 2002; Kane & Garber, 2009), including internalizing symptoms; cortisol reactivity may play a role in mediating these associations. In light of the trend for fathers to be increasingly involved in child rearing (NICHD, 2000), additional work on the role on paternal depression in children’s early emerging depression is clearly needed.
It is important to note that a family history of depression is a marker of a diverse array of processes that can increase children’s depression risk, including both intrinsic (e.g., genetic) and extrinsic (e.g., environmental) factors, including negative parenting practices (Lovejoy et al., 2000). In Study 2, observational ratings of negative parenting behavior were collected at baseline; as these ratings were almost entirely based on maternal behavior, we examined whether the same pattern of interaction was obtained for the single analysis in which maternal depression interacted with children’s cognitive risk (i.e., the analysis presented in Table 4) when substituting hostile and intrusive parenting for maternal depression in models. These interaction terms were not significant; thus, we cannot say that the effect of maternal depression on children’s cortisol reactivity can be better understood as a marker of poor parenting. However, as we did not collect data on fathers’ interactions with their children, we cannot rule out the possibility that the effect of paternal depression we found is a proxy for the negative impact of paternal depression on fathers’ caregiving (Wilson & Durbin, 2010). Certainly, it would stand to reason that children with heightened negative cognition (e.g., self-blaming attributions, attention and memory biases favoring negative stimuli) would be particularly susceptible to poor caregiving in terms of its impact on cortisol reactivity. Along similar lines, given the associations between depression and relationship discord, it is also possible that parental depression is a marker of parental relationship discord, which may also influence children’s cortisol reactivity (Davies, Sturge-Apple, & Cicchetti, 2011). Thus, future work should aim to more clearly delineate specific processes that interact with children’s cognitive risk to produce increased depression vulnerability.
It would be premature to apply our findings to preventative efforts. However, should future studies replicate the pattern of findings we obtained, fine-grain preventions might be developed targeting children with parental depression who also evince elevated cognitive risk, with the focus of increasing these high children’s coping skills and stress reduction. Prevention studies using family-based cognitive interventions in the context of parental depression that have focused on enhancing youth coping (Compas et al., 2010) are broadly complementary to this suggestion. Furthermore, previous work has shown that disengagement from negative emotional information may decrease cortisol reactivity (Ellenbogen, Hodgins, Walker, Couture, & Adam, 2006). This may suggest that some of the risk associated with the presence of both familial depression and heightened cognitive vulnerability can be attenuated by training children in strategies that reduce the tendency to preferentially process negative information in their environments, or by targeting children’s coping strategies for dealing with stress.
We have framed heightened cortisol reactivity to stress as a marker of depression risk. While there is evidence to support this assertion, it is also important to note that cortisol reactivity may not be a marker of risk, but of contextual sensitivity more broadly speaking (Belsky & Pluess, 2009). According to the notion of differential susceptibility, heightened cortisol reactivity is an index of how likely an individual is to react both positive and negative environmental circumstances; if so, the long-term implications of heightened cortisol reactivity for children’s adaptation and maladaptation can only be understood in terms of the broader environmental context. Previous work also implicates certain genetic variants as markers of differential susceptibility (Hankin et al., 2011; Hayden et al., 2010; 2013); given the heritable basis of cortisol reactivity (e.g., Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2013), genetic markers could be yet another important level of analyses in models of cortisol function. Further, both hyper- and hyporeactivity of the cortisol response have been linked to psychopathology risk (e.g., see Hankin et al., 2010, for evidence in depression). Further longitudinal work is therefore needed to examine the long-term outcomes related to different patterns of children’s cortisol reactivity to stress.
Our study had a number of strengths. First, we examined interactions between cognitive risk and parental depression in two independent samples. We used multiple, diverse measures of children’s cognitive risk and structured clinical interviews to assess parental depression. We collected an impressive number of cortisol samples for research of this kind, permitting a fuller characterization of children’s HPA axis reactivity to stress, and took steps to ensure that an accurate baseline sample was obtained. We examined the interactive effects of two well established markers of depression risk across theoretically relevant levels of analysis, a surprisingly under-investigated topic in the developmental psychopathology of depression (see Hankin, 2012, for a theoretical model emphasizing this approach). For Study 2, the interval between our follow-ups was lengthy for research of this kind, providing a stringent test of the relationship between children’s cognitive risk, parental psychiatric history, and cortisol reactivity in middle childhood, and our attrition rates were relatively low.
However, our study also had some weaknesses. Due to funding constraints and to reduce the burden on participants, clinical interview data were collected only on primary caregivers in Study 1; similarly, in Study 2, we collected measures of parental psychopathology at baseline only. Thus, it is possible that we missed new cases of parental depression that occurred during the two-year follow-up period. Additionally, our outcome variable was cortisol reactivity, not depression per se. Hence, further longitudinal work is needed to demonstrate that the multi-level model of risk to internalizing distress we have proposed, by which interaction between risks lead to heightened cortisol reactivity, in turn eventuates in depression or other emotional distress disorders. While we have framed our study in terms of predicting children’s depression risk, heightened cortisol reactivity is also relevant to anxiety disorders (e.g., Roelofs et al., 2009). While this does not negate the importance of our findings, it will be important for future research to investigate the specificity of these effects to youth depression relative to anxiety to further evaluate the applicability to the pathogenesis of anxiety as well as depression. We did not collect measures of children’s stressful life events at baseline, which may also interact with cognitive risk and familial depression to predict cortisol reactivity, and we were unable to control for children’s baseline cortisol reactivity due to the lack of tasks that are known to elicit comparable patterns of cortisol reactivity across different child ages. We ran multiple analyses without correction, increasing the likelihood that some associations found were due to chance and will not be replicated in future tests of interplay between parent depression and child cognitive risk. However, as so little is known in the current literature about interactions between aspects of children’s depression risk, we felt a more exploratory approach to data analysis was warranted to lay the groundwork for subsequent investigations of this important topic.
In conclusion, we found in two separate samples of children that aspects of cognitive risk for depression interacted with parental depression, especially paternal depression, to predict increased cortisol reactivity to stress in middle childhood. Our findings indicate that future attempts to model children’s emerging depression risk may benefit from the examination of interactions between multiple sources of vulnerability. Further, including measures of paternal depression also appears important toward generating a fuller picture of the dynamic nature of children’s emerging stress reactivity and depression risk.
Figure 4.
Interaction between paternal depression history and child SRET-N predicting AUCg
Note: SRET-N- Self-referent encoding task negative processing; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Table 6.
Regression testing the interaction between paternal depression history and child SRET-N predicting AUCg
| Overall Model | Change Statistics | ||||||
|---|---|---|---|---|---|---|---|
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 5, 152 | .07 | 2.36* | ||||
| Parental AD | .14† | ||||||
| DSRS | −.13 | ||||||
| SRET-N | −.03 | ||||||
| MDD | −.01 | ||||||
| PDD | .19* | ||||||
| Step 2 | 6, 151 | .11 | 2.94* | 1,151 | .03 | 5.51* | |
| Parental AD | .14† | ||||||
| DSRS | −.18* | ||||||
| SRET-N | −.12 | ||||||
| MDD | .02 | ||||||
| PDD | .17* | ||||||
| SRET-N X PDD | .22* | ||||||
p < .01;
p < .05;
p < .10.
Note: Parental AD-parental history of any anxiety disorder; DSRS-Depression Self-Rating Scale; SRET-N- Self-referent encoding task negative processing; MDD-maternal depressive disorder; PDD-paternal depressive disorder; AUCg-Area under the curve-ground.
Acknowledgments
This research was supported by an Early Researcher Award from the Ontario Ministry of Research and Innovation (Hayden), a SSHRC Standard Research Grant (Hayden), an NIMH grant 5R01 MH077195 (Hankin), and a Career Development Award from the National Institute of Mental Health K01 MH092603 (Olino). The Ontario Mental Health Foundation and the Children’s Health Research Institute provided student support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
Contributor Information
Elizabeth P. Hayden, Western University
Benjamin L. Hankin, University of Denver
Sarah V.M. Mackrell, Western University
Haroon I. Sheikh, Western University
Patricia L. Jordan, Western University
David J.A. Dozois, Western University
Shiva M. Singh, Western University
Thomas M. Olino, University of Pittsburgh Medical Center
Lisa S. Badanes, Metropolitan State University of Denver
References
- Abela JR. The hopelessness theory of depression: A test of the diathesis-stress and causal mediation components in third and seventh grade children. Journal of Abnormal Child Psychology. 2001;29:241–254. doi: 10.1023/a:1010333815728. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology perspective. New York, NY: Guilford Press; 2008. [Google Scholar]
- Abramson LY, Metalsky FI, Alloy LB. Hopelessness depression: A theory based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Archives of General Psychiatry. 1997;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Carlson GA. The Depression Self-Rating Scale: Utility with child psychiatric inpatients. Journal of Consulting and Clinical Psychology. 1985;53:491–499. doi: 10.1037//0022-006x.53.4.491. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G. Maternal depression, infant psychobiological development, and risk for depression. In: Goodman SH, Gotlib IH, editors. Children of depressed parents: Mechanisms of risk and implications for treatment. Washington, DC: American Psychological Association; 2002. pp. 37–58. [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Azar R, Paquette D, Zoccolillo M, Baltzer F, Tremblay RE. The association of major depression, conduct disorder, and maternal overcontrol with a failure to show a cortisol buffered response in four-month-old infants of teenage mothers. Biological Psychiatry. 2007;62:573–579. doi: 10.1016/j.biopsych.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker ED, Copeland E, Maughan B, Jaffee SR, Uher R. Relative impact of maternal depression and associated risk factors on offspring psychopathology. The British Journal of Psychiatry. 2012;200:124–129. doi: 10.1192/bjp.bp.111.092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. The American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Birleson P. The validity of depressive disorder in childhood and the development of a self□rating scale: A research report. Journal of Child Psychology and Psychiatry. 1981;22:73–88. doi: 10.1111/j.1469-7610.1981.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Pargas EF, Walker PG, Mewport DJ, Stowe Z. Maternal depression and infant cortisol: Influences of timing, comorbidity and treatment. Journal of Child Psychology and Psychiatry. 2008;49:1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner E. Mood induction in children: Methodological issues and clinical implications. Review of General Psychology. 2000;4:264–283. [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer DH. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus C. Stepmom [Motion picture] United States: Sony Pictures; 1998. (Director) [Google Scholar]
- Compas BE, Champion JE, Forehand R, Cole DA, Reeslund KL, Roberts L. Coping and parenting: Mediators of 12-month outcomes of a family group cognitive-behavioral preventive intervention with families of depressed parents. Journal of Consulting and Clinical Psychology. 2010;78:623–634. doi: 10.1037/a0020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Differences in patterns of processing bias for emotional information across disorders: An investigation of attention, memory and prospective cognition in children and adolescents with depression, generalized anxiety and Posttraumatic Stress Disorder (PTSD) Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Review. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D. Interparental aggression and children's adrenocortical reactivity: Testing an evolutionary model of allostatic load. Development and Psychopathology. 2011;23:801–814. doi: 10.1017/S0954579411000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Khun C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67:63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. The Association for Clinical Biochemistry. 2007;44:281–284. doi: 10.1258/000456307780480954. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-aged offspring of depressed parents: Moderation by early parenting. Psychological Science. 2011;22:650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4th ed. Minneapolis, MN: NCS Pearson Inc; 2007. [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker C-D, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;10:1164–1180. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente MA, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR axis I disorders, research version, non-patient edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- Garber J, Flynn C. Predictors of depressive cognitions in young adolescents. Cognitive Therapy and Research. 2001;25:353–376. [Google Scholar]
- Garber J, Gallerani CM, Frankel SA. Depression in children. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. New York, NY: Guilford Press; 2009. pp. 405–443. [Google Scholar]
- Garber J, Martin NC. Negative cognitions in offspring of depressed parents: Mechanisms of risk. In: Goodman SH, Gotlib IH, editors. Children of depressed parents: Mechanisms of risk and implications for treatment. Washington, DC: American Psychological Association; 2002. pp. 121–153. [Google Scholar]
- Gencoz T, Voels ZR, Gencoz F, Pettit JW, Joiner TE., Jr Specificity of information processing styles to depressive symptoms in youth psychiatric inpatients. Journal of Abnormal Child Psychology. 2000;29:255–262. doi: 10.1023/a:1010385832566. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR, editors. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology. New York, NY: Cambridge University Press; 2005. pp. 343–356. [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stress paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C. Adolescent depression: Stressful interpersonal contexts and risk for recurrence. Current Directions in Psychological Science. 2009;18:200–204. doi: 10.1111/j.1467-8721.2009.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Zupan BA. Self-schemas, depression, and the processing of personal information in children. Journal of Experimental Child Psychology. 1984;37:598–608. doi: 10.1016/0022-0965(84)90079-1. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Future directions in vulnerability to depression among youth: Integrating risks across multiple levels of analysis. Journal of Child and Adolescent Clinical Psychology. 2012;41:695–718. doi: 10.1080/15374416.2012.711708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Oppenheimer C, Jenness J, Barrocas A, Shapero BG, Goldband J. Developmental origins of cognitive vulnerabilities to depression: Review of processes contributing to stability and change across time. Journal of Clinical Psychology. 2009;65:1327–1338. doi: 10.1002/jclp.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Snyder HR, Gulley LD. Cognitive risks in developmental psychopathology. In: Cicchetti D, editor. Developmental Psychopathology. 3rd ed. New York, NY: Wiley; 2013. [Google Scholar]
- Hayden EP, Olino TM, Bufferd SJ, Miller A, Dougherty LR, Sheikh HI, Klein DN. The serotonin transporter linked polymorphic region and brain-derived neurotrophic factor valine to methionine at position 66 polymorphisms and maternal history of depression: Associations with cognitive vulnerability to depression in childhood. Development and Psychopathology. 2013;25:587–598. doi: 10.1017/S0954579413000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Dyson MW, Durbin CE, Sheikh HI, Singh SM. The Role of Brain-Derived Neurotrophic Factor Genotype, Parental Depression, and Relationship Discord in Predicting Early-Emerging Negative Emotionality. Psychological Science. 2010;21:1678–1685. doi: 10.1177/0956797610385357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, Olino TM. Positive emotionality at age three predicts cognitive styles in seven-year-old children. Development & Psychopathology. 2006;18:409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Heim-Dreger U, Kohlmann CW, Eschenbeck H, Burkhardt U. Attentional biases for threatening faces in children: Vigilant and avoidance processes. Emotion. 2006;6:320–325. doi: 10.1037/1528-3542.6.2.320. 10.1037/1528-3542.6.2.320. [DOI] [PubMed] [Google Scholar]
- Hoferl M, Krist S, Buchbauer G. Adaptation of DELFIA cortisol kit for determination of salivary cortisol concentration. Pharmaceutical & Medicinal Chemistry. 2005;338:493–497. doi: 10.1002/ardp.200500116. [DOI] [PubMed] [Google Scholar]
- Hunt C, Keogh E, French CC. Anxiety sensitivity, conscious awareness and selective attentional biases in children. Behaviour Research and Therapy. 2007;45:497–509. doi: 10.1016/j.brat.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Gillberg C, Arvidsson T, Broberg AG. The youth self-report (YSR) and the depression self-rating scale (DSRS) as measures of depression and suicidality among adolescents. European Child and Adolescent Psychiatry. 2002;11:31–37. doi: 10.1007/s007870200005. [DOI] [PubMed] [Google Scholar]
- Jacobs RH, Reinecke MA, Gollan JK, Kane P. Empirical evidence of cognitive vulnerability for depression among children and adolescents: A cognitive science and developmental perspective. Clinical Psychology Review. 2008;28:759–782. doi: 10.1016/j.cpr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Jansen MA, van der Gaag RJ, Matthys W, van Engeland H. Pituitary-adrenal reactivity in a child psychiatric population: Salivary cortisol response to stressors. European Neuropsychopharmacology. 1999;9:67–75. doi: 10.1016/s0924-977x(98)00003-0. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Joorman J, Gotlib IH. Does processing of emotional stimuli predict symptomatic improvement and diagnostic recovery from major depression? Emotion. 2007;7:201–206. doi: 10.1037/1528-3542.7.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. New York, NY: Guilford Press; 2009. pp. 298–321. [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kane PP, Garber J. Parental depression and child externalizing and internalizing symptoms: Unique effects of fathers’ symptoms and perceived conflict as a mediator. Journal of Child and Family Studies. 2009;18:465–472. [Google Scholar]
- Kashani JH, Reid JC, Rosenberg TK. Levels of hopelessness in children and adolescents: a developmental perspective. Journal of Consulting and Clinical Psychology. 1989;57:496–499. doi: 10.1037//0022-006x.57.4.496. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Archives of General Psyhciatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression and the processing of emotional stimuli in non-referred girls and boys. Behavioral Sciences and the Law. 2006;24:21–37. doi: 10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- Klein DN, Dougherty LR, Olino TM. Toward guidelines for evidence-based assessment of depression in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:412–432. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- Klein DN, Riso LP, Donaldson SK, Schwartz JE, Anderson RL, Ouimette PC, Aronson TA. Family study of early-onset dysthymia: Mood and personality disorders in relatives of outpatients with dysthymia and episodic major depression and normal controls. Archives of General Psychiatry. 1995;52:487–496. [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Rohde P, Seeley JR. Family study of chronic depression in a community sample of young adults. American Journal of Psychiatry. 2004;161:646–653. doi: 10.1176/appi.ajp.161.4.646. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Rose S. Ten-year prospective follow-up study of the naturalistic course of dysthymic disorder and double depression. American Journal of Psychiatry. 2006;163:872–880. doi: 10.1176/ajp.2006.163.5.872. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kryski KR, Smith HJ, Sheikh HI, Singh SM, Hayden EP. Assessing stress reactivity indexed via salivary cortisol in preschool-aged children. Psychoneuroendocrinology. 2011;36:1127–1136. doi: 10.1016/j.psyneuen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Holzhauer S, Huffziger S. Decreased cortisol response to awakening is associated with cognitive vulnerability to depression in a nonclinical sample of young adults. Psychoneuroendocrinology. 2007;32:199–209. doi: 10.1016/j.psyneuen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Huffziger S, Liebsch K. Rumination, distraction and mindful self-focus: effects on mood, dysfunctional attitudes and the cortisol stress response. Psychological Medicine. 2009;39:219–228. doi: 10.1017/S0033291708003553. [DOI] [PubMed] [Google Scholar]
- Kuiper NA, Derry PA. Depressed and nondepressed content self-reference in mild depressives. Journal of Personality. 1982;50:67–80. doi: 10.1111/j.1467-6494.1982.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Lakdawalla Z, Hankin BL, Mermelstein R. Cognitive theories of depression in children and adolescents: A conceptual and quantitative review. Clinical Child and Family Psychology Review. 2007;10:1–24. doi: 10.1007/s10567-006-0013-1. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical manual and affective ratings. 1997 NIMH Center for the Study of Emotion and Attention. [Google Scholar]
- Laurent HK, Leve LD, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D. Effects of parental depressive symptoms on child adjustment moderated by hypothalamic pituitary adrenal activity: within- and between-family risk. Child Development. 2013;84:528–542. doi: 10.1111/j.1467-8624.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF. Genetics of Major Depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. New York, NY: Guilford Press; 2009. pp. 165–186. [Google Scholar]
- Lopez-Duran NL, Kovacs M, George C. Hypothalamic pituitary adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews: Neuroscience. 2009;10:434–345. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. Quarterly Journal of Experimental Psychology. 1988;40:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vazquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review. 1992;12:227–255. [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transported gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: A Meta-analysis. Molecular Psychiatry. 2013;18:1018–1024. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Factors associated with fathers' caregiving activities and sensitivity with young children. Journal of Family Psychology. 2000;14:200–219. [PubMed] [Google Scholar]
- Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31:1583–1591. doi: 10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. The American Psychologist. 1993;48:155–168. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Roelofs K, van Peer J, Berretty E, de Jong P, Spinhoven P, Elzinga BM. Hypothalamus-Pituitary-Adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biological Psychiatry. 2009;65:336–343. doi: 10.1016/j.biopsych.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime [computer software] Pittsburgh: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Statistics Canada. 2006 Community Profiles, London, Ontario, 2006 Census. 2007 (Catalogue no. 92–591-XWE). Retrieved from the Statistics Canada website http://www12.statcan.ca/census-recensement/2006/dp-pd/prof/92-591/index.cfm?Lang=E.
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. The American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. Journal of Abnormal Psychology. 1999;108:202–210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Dent J. Cognitive vulnerability to depression: An investigation of two hypotheses. British Journal of Clinical Psychology. 1987;26:113–126. doi: 10.1111/j.2044-8260.1987.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Parker SW, Liu C. Individual Differences in Cardiac and HPA Reactivity to a Psychological Stressor; Poster presented at the Society for Research in Child Development Bi-annual Meeting, Minneapolis.Apr, 2001. [Google Scholar]
- van Santen A, Vreeburg SA, Van der Does AJ, Spinhoven P, Zitman FG, Penninx BW. Psychological traits and the cortisol awakening response: Results from the Netherland Study of Depression and Anxiety. Psychoneuroendocrinology. 2011;36:240–248. doi: 10.1016/j.psyneuen.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Wallace J, Schnieder T, McGuffin P. Genetics of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York, NY: Guilford Press; 2002. pp. 169–191. [Google Scholar]
- Wilson S, Durbin CE. Effects of paternal depression on fathers' parenting behaviors: A meta-analytic review. Clinical Psychology Review. 2010;30:167–180. doi: 10.1016/j.cpr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Joormann J. Stress reactivity in social anxiety disorder with and without comorbid depression. Journal of Abnormal Psychology. 2012;121:250–255. doi: 10.1037/a0025079. [DOI] [PubMed] [Google Scholar]
- Zieff H. My Girl [Motion picture] United States: Sony Pictures; 1991. (Director) [Google Scholar]
- Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research. 2012;73:1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]