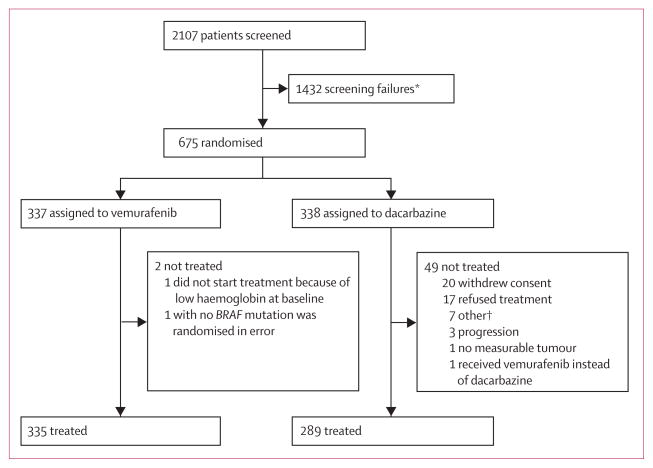
Figure 1. Trial profile.
*Most common reason for screen failure included a negative cobas test for the BRAF mutation (1086 patients; 76%), brain metastases (110 patients; 8%), deterioration/ECOG status/death (46 patients; 3%). †Pulmonary embolism before treatment (one patient), not eligible per exclusion criteria (one patient), clinical deterioration (one patient), brain metastases (two patients), and two patients pending resolution on treatment status.