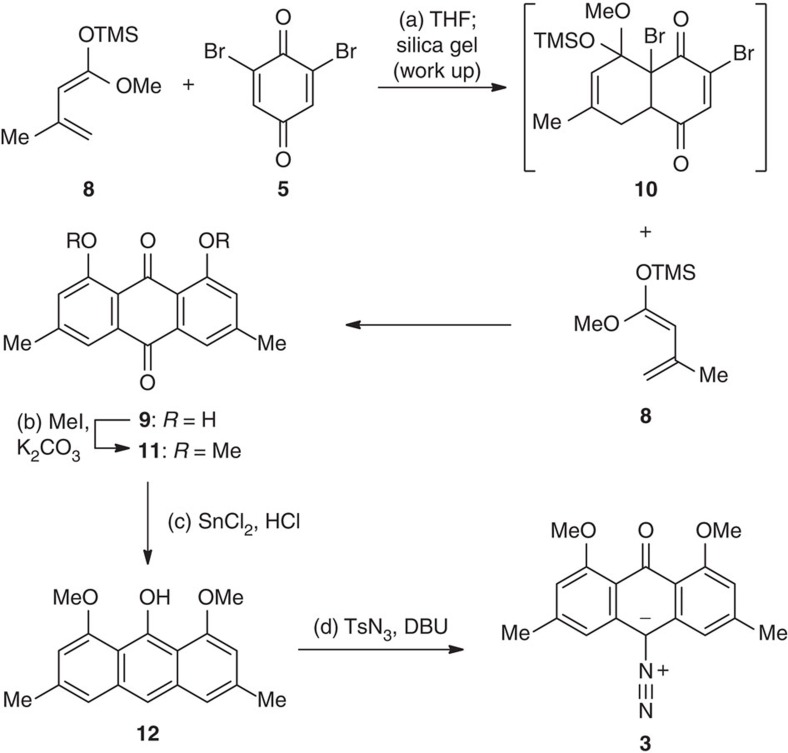
Figure 2. Synthesis of the anthraquinone segment.
Reagents and conditions: (a) tetrahydrofuran (THF), −78 °C, 6 h; then silica gel, 22 °C, 2 h. (b) K2CO3 (3.0 eq), MeI (2.5 eq), N,N-dimethylformamide (DMF), 22 °C, 18 h, 45% (two steps). (c) SnCl2·H2O (6.0 eq), aqueous HCl (37 wt%), AcOH, 22 °C, 30 min. (d) DBU (3.0 eq), TsN3 (1.1 eq), methylene chloride (CH2Cl2), 22 °C, 20 h, 89% (two steps). TMS, trimethylsilyl.