Abstract
Electrochemical water splitting has been considered as a promising approach to produce clean and sustainable hydrogen fuel. However, the lack of high-performance and low-cost electrocatalysts for hydrogen evolution reaction hinders the large-scale application. As a new class of porous materials with tunable structure and composition, metal-organic frameworks have been considered as promising candidates to synthesize various functional materials. Here we demonstrate a metal-organic frameworks-assisted strategy for synthesizing nanostructured transition metal carbides based on the confined carburization in metal-organic frameworks matrix. Starting from a compound consisting of copper-based metal-organic frameworks host and molybdenum-based polyoxometalates guest, mesoporous molybdenum carbide nano-octahedrons composed of ultrafine nanocrystallites are successfully prepared as a proof of concept, which exhibit remarkable electrocatalytic performance for hydrogen production from both acidic and basic solutions. The present study provides some guidelines for the design and synthesis of nanostructured electrocatalysts.
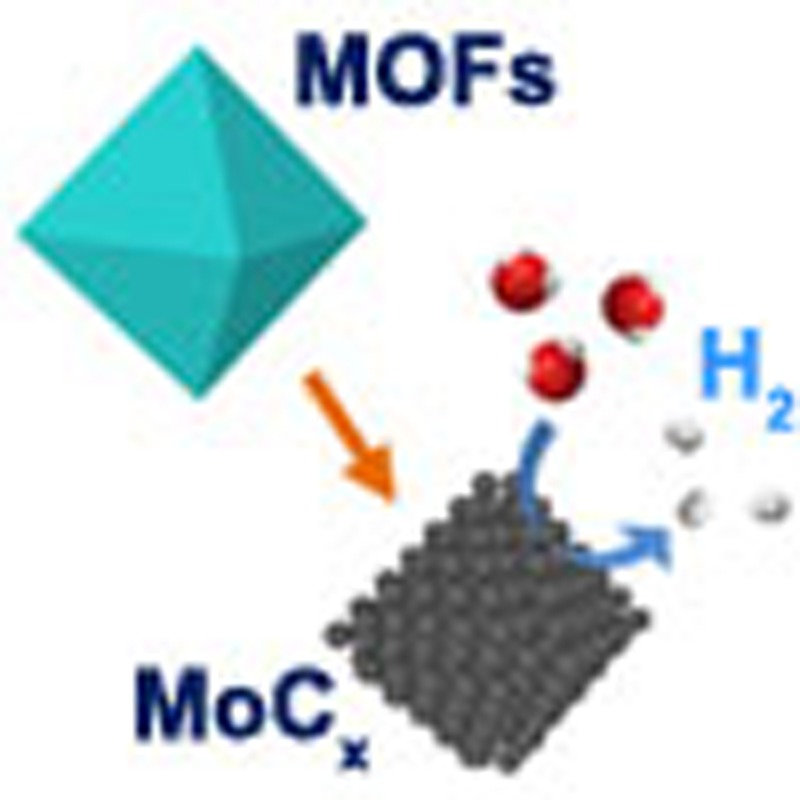 There is extensive research into non-platinum electrocatalysts for hydrogen evolution. Here, the authors report a molybdenum carbide catalyst, prepared via the carburization of a copper metal-organic framework host/molybdenum-based polyoxometalates guest system, and demonstrate its catalytic activity.
There is extensive research into non-platinum electrocatalysts for hydrogen evolution. Here, the authors report a molybdenum carbide catalyst, prepared via the carburization of a copper metal-organic framework host/molybdenum-based polyoxometalates guest system, and demonstrate its catalytic activity.
The rapid growth of global energy consumption and the associated environmental issues have triggered the urgent demand for renewable and clean energy sources. Electrochemical water splitting driven by solar energy has been considered as an attractive approach to produce hydrogen (H2) fuel, a sustainable, secure and environmentally benign energy vector1,2,3. Efficient water splitting requires high-performance electrocatalysts to promote the hydrogen evolution reaction (HER). Platinum (Pt) has been identified as the most active HER catalyst, whereas its high cost and low abundance hinder the large-scale application4. Therefore, numerous efforts have been devoted to search for noble metal-free HER catalysts5,6,7,8,9,10,11,12,13,14,15,16,17,18. Transition metal carbides, such as molybdenum (Mo) and tungsten (W) carbides have been under investigation for decades in the fields of catalysis in view of their high similarity to Pt-group metals19,20, and have been recently suggested as promising electrocatalysts for HER21,22. Particularly, β-phase molybdenum carbide (β-Mo2C) has been demonstrated as a highly active HER catalyst even as bulky particles23, and the performance could be further improved by constructing proper nanostructures24,25,26,27. However, controllable synthesis of nanostructured metal carbides with small nanocrystallites and desirable porosity towards high electrocatalytic activity still remains as a great challenge, due to the difficulty to achieve uniform carburization and the inevitable coalescence of nanoparticles at high reaction temperature.
In recent years, metal-organic frameworks (MOFs) have emerged as a new class of porous materials with widespread applications in gas storage/separation, catalysis, sensing and drug delivery28,29,30. Moreover, syntheses of functional materials from MOFs have drawn fast-growing interests as well. The periodically porous and hybrid structure of MOFs offer unique benefits for the fabrication of carbon and/or metal-based nanostructured materials. Specifically, MOFs-derived porous carbon, metal oxides, metal/carbon and metal oxide/carbon nanocomposites have been reported by using MOFs as both the precursor and template31,32,33,34,35,36. For example, MOFs-derived nanoporous carbon materials exhibit exceptionally high surface area and uniform porosity, which are largely originated from the ordered and porous structure of MOFs32,33,37. We have previously used Prussian blue cubic microcrystals to prepare various iron oxide-based hollow microboxes with complex shell structures and compositions38,39. This category of MOFs-derived materials has been recently extended to iron carbide40. However, in most of these studies, MOFs are exclusively used as the sole precursor. Although the huge family of MOFs has covered a wide range of metal species, these conventional MOFs-derived strategies are mainly based on a limited number of MOFs that are easily obtainable and/or with controllable morphology. Consequently, the reported MOFs-derived metal-based materials are typically limited to a handful of elements (for example, Zn, Cu, Co, Fe and so on).
In this work, we develop a MOFs-assisted strategy for synthesizing porous molybdenum carbide octahedral nanoparticles (denoted as MoCx nano-octahedrons) that consist of very small nanocrystallites as electrocatalysts for efficient hydrogen production. Distinct from previous studies, the present synthesis strategy relies on the in situ and confined carburization reaction between the organic ligands (or their derived carbon-based species) of MOFs and guest polyoxometalates (POMs) that reside in the pores of the MOFs host. The introduction of guest metal species into the MOFs host as co-precursor enables easy synthesis of early transition metal (for example, Mo, W and V) carbides, which are difficult to obtain from a single MOFs source. Meanwhile, these non-coordinating POMs are also uniformly distributed and surrounded by organic ligands in atomic scale, thus guaranteeing in situ and homogeneous carburization reaction that produces small carbide nanocrystallites. In addition, the carburization process would be confined within the carbonaceous matrix derived from organic ligands of MOFs, which effectively prevents the agglomeration and coalescence of in situ-generated carbide nanocrystallites. As a proof of concept, we demonstrate the synthesis of molybdenum carbide using this MOFs-assisted approach in view of its promising application in catalysis (for example, for HER) and the easy encapsulation of Mo-based POMs in a particular MOFs host as discussed shortly. Interestingly, the as-prepared molybdenum carbide is in a η-MoC phase, which is unexpected at a relatively low carburization temperature of 800 °C and has not been well investigated for electrocatalytic hydrogen production41. Benefiting from the porous and robust structure, as well as the ultrafine primary nanocrystallites, the as-prepared molybdenum carbide nano-octahedrons exhibit remarkable electrocatalytic activity for HER in both acidic and basic conditions. The present strategy is also applicable to synthesize W and Mo-W carbides, and could be extended to other early transition metals as well.
Results
MOFs-assisted synthesis strategy for molybdenum carbide
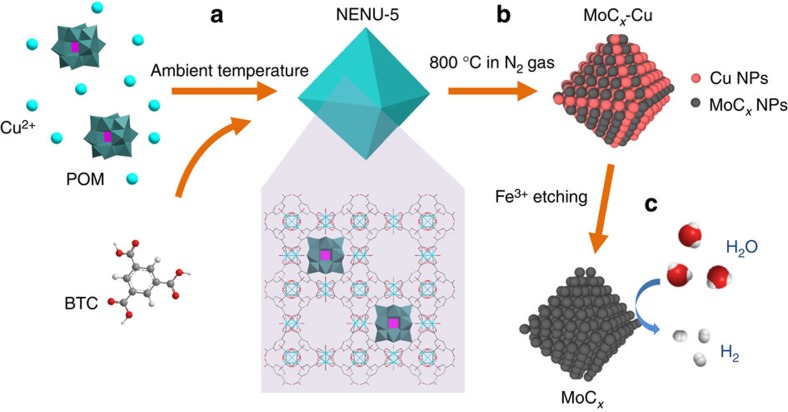
The overall synthesis route to prepare porous MoCx octahedral sub-micrometre-sized particles (denoted as MoCx nano-octahedrons) as efficient HER catalysts is illustrated in Fig. 1. We choose a unique MOFs-based compound as the precursor with a formula of [Cu2(BTC)4/3(H2O)2]6[H3PMo12O40] (NENU-5; BTC=benzene-1,3,5-tricarboxylate), which is based on a well-studied Cu-based MOF [HKUST-1; Cu3(BTC)2(H2O)3] with Mo-based Keggin-type POMs (H3PMo12O40) periodically occupying the largest pores42. In this work, NENU-5 nano-octahedrons are synthesized by a facile and scalable co-precipitation method at ambient temperature. The as-prepared NENU-5 nano-octahedrons containing substantial amount of Mo are directly heated at 800 °C in N2 gas flow to produce MoCx-Cu. During the annealing process, the Mo-based POMs react with carbonaceous species derived from BTC ligands to form MoCx nanocrystallites, meanwhile Cu2+ clusters are reduced to metallic Cu. Finally, MoCx nano-octahedrons composed of small nanocrytallites are obtained by etching the metallic Cu nanoparticles with aqueous solution of FeCl3 (2 Fe3++Cu→Cu2++2 Fe2+), and used as electrocatalysts for HER.
Figure 1. Schematic illustration of the synthesis procedure for porous MoCx nano-octahedrons.
(a) Synthesis of NENU-5 nano-octahedrons with Mo-based POMs residing in the pores of HKUST-1 host. (b) Formation of MoCx-Cu nano-octahedrons after annealing at 800 °C. (c) Removal of metallic Cu nanoparticles by Fe3+ etching to produce porous MoCx nano-octahedrons for electrocatalytic hydrogen production.
Synthesis of porous MoC x nano-octahedrons
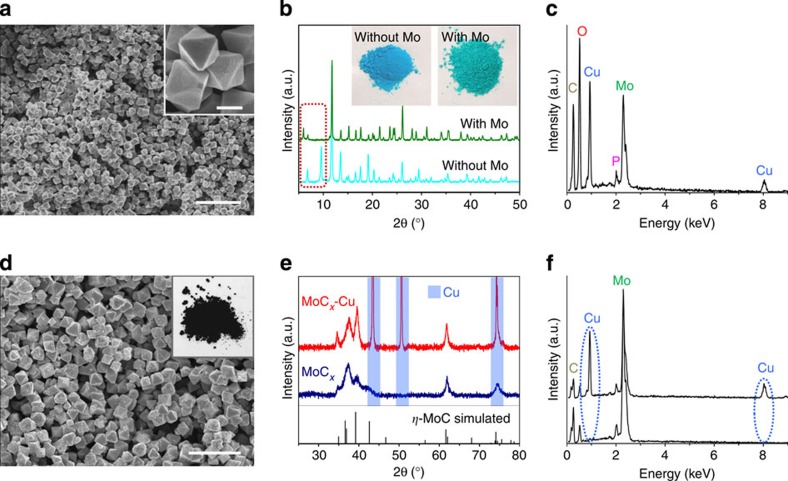
Figure 2a shows typical field-emission scanning electron microscopy (FESEM) images of the as-prepared NENU-5 particles in octahedral or slightly truncated octahedral shape with a sub-micrometre size of ~800 nm. The size of the as-prepared NENU-5 particles can be easily tuned by varying the addition amount of L-glutamic acid that slows down the nucleation rate (Supplementary Fig. 1). Powder X-ray diffraction (XRD) pattern shown in Fig. 2b (upper curve) confirms the phase purity as well as the excellent crystallinity of the NENU-5 nano-octahedrons. The XRD pattern of HKUST-1 is also provided for comparison (lower curve in Fig. 2b), which exhibits different diffraction peaks especially below 10°. Moreover, the successful incorporation of Mo-based POMs in the HKUST-1 framework can be visually verified by the green colour of the product, distinct from the blue colour of pristine HKUST-1 (insets of Fig. 2b)43. The chemical composition is further examined by energy-dispersive X-ray spectroscopy (EDX). The spectrum (Fig. 2c) evidently shows the presence of substantial amount of Mo in the as-prepared NENU-5 nano-octahedrons. Moreover, a Mo/Cu atomic ratio of ~0.7 suggested by EDX quantitative analysis is lower than the theoretical value of 1 in NENU-5. Therefore, some vacancies of POMs exist in the as-prepared NENU-5 nano-octahedrons, which are probably due to the low synthesis temperature and/or the blocking of pores by other species from the solution. Such vacancies are also responsible for the low intensity of characteristic XRD peaks in small angle region43. Moreover, the vacancies of POMs would reduce the yield of MoCx and possibly result in less uniform distribution of MoCx nanocrystallites, which should be minimized in future studies.
Figure 2. Characterizations of precursors and MoCx nano-octahedrons.
(a) FESEM image (inset: magnified image; scale bar, 500 nm) of the as-prepared NENU-5 nano-octahedrons; scale bar, 5 μm. (b) XRD patterns (inset: digital photos) of NENU-5 (with Mo) and HKUST-1 (without Mo). (c) EDX spectrum of the as-prepared NENU-5 nano-octahedrons. (d) FESEM image (inset: digital photo) of porous MoCx nano-octahedrons; scale bar, 2 μm. (e) XRD patterns and (f) EDX spectrums of MoCx-Cu and MoCx nano-octahedrons.
After the annealing and etching processes, the MoCx sample is obtained as a black powder (inset of Fig. 2d). FESEM image (Fig. 2d) reveals that the octahedral shape of the particles is well retained, while the surface becomes slightly rougher. The complete removal of Cu particles by Fe3+ etching is confirmed by both XRD and EDX analyses. As shown in Fig. 2e, the three strong and sharp peaks from metallic Cu in the XRD pattern of MoCx-Cu sample (upper curve) are no longer observed in the pattern of MoCx sample (lower curve). Moreover, EDX spectrum of the MoCx sample confirms the main composition of Mo and C, and also excludes the presence of Cu (Fig. 2f). Surprisingly, the XRD pattern of MoCx sample shows distinct results from previous reports that typically produce β-Mo2C by annealing mixtures of molybdenum salts and organic compounds at similar temperatures25,26,27,44. The pattern can be satisfactorily assigned to hexagonal η-MoC phase (Supplementary Fig. 2) that is usually produced at much higher temperatures45,46 or in the presence of NiI2 with short reaction duration41. In our system, the η-MoC phase can be produced between 750 and 850 °C with little alteration in the XRD patterns (Supplementary Fig. 3). This unusual result implies the distinct characteristics of chemical reactions confined in the MOFs matrix, which is also exemplified by the uncommon synthesis of pure brookite-phase TiO2 through replication of MOFs47. Considering the likelihood to form substoichiometric η-MoC1−x phases46, and the difficulty to determine the exact chemical composition due to the presence of extra amorphous carbon (as discussed shortly), the as-prepared molybdenum carbide is denoted as MoCx in this work. Moreover, the diffraction peaks are significantly broadened, suggesting the very small size of nanocrystallites due to the effective inhibition of coalescence and crystal growth during the confined carburization process. Meanwhile, excessive growth of Cu particles in the MoCx-Cu sample as suggested by the XRD pattern (see upper curve in Fig. 2e) still occurs during the annealing process, which is probably related to the relatively low melting point of Cu.
Structural characterizations of MoC x nano-octahedrons
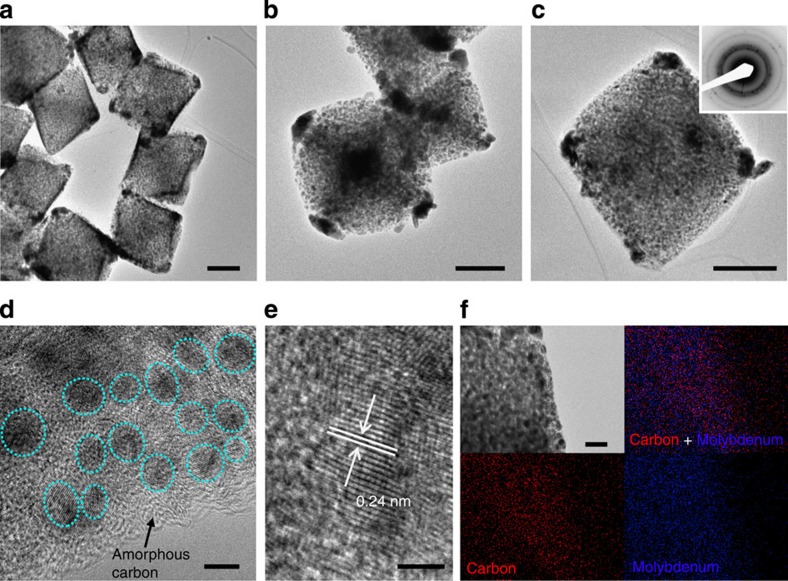
The structure of the as-prepared MoCx nano-octahedrons is further examined by transmission electron microscopy (TEM) as depicted in Fig. 3. The sample appears as rhombic or cubic particles under TEM observation as the projections of octahedral particles from different directions (Fig. 3a–c). A closer examination on the MoCx nano-octahedrons reveals the highly porous texture throughout the whole particle. Each MoCx nano-octahedron is composed of numerous small nanocrystallites, and the polycrystalline nature is confirmed by selected-area electron diffraction (SAED) pattern as shown in the inset of Fig. 3c. An interesting observation is that some large particles appear at the corners of the octahedral particles. This is probably due to the higher surface activity and stress in these regions that cause easy collapse of the MOFs matrix and subsequent growth of MoCx nanocrystallites during the high-temperature reaction. Indeed, our initial attempt to prepare MoCx using much smaller NENU-5 nanoparticles (shown in Supplementary Fig. 1a) with high surface activity results in strongly aggregated particles with a poorly crystallized β-Mo2C phase that cannot be well dispersed into suspension for subsequent electrochemical measurements (Supplementary Fig. 4). Therefore, a robust secondary structure with a moderate size (for example, sub-micrometre-sized NENU-5 particles) is essential to successfully carry out the confined carburization reaction while providing large exposed surface for catalytic purpose.
Figure 3. Characterizations of MoCx nano-octahedrons.
(a–c) TEM images (scale bar, 200 nm; inset of c: SAED pattern), (d) magnified TEM image (scale bar, 5 nm), (e) HRTEM image (scale bar, 2 nm) and (f) elemental mapping (red: carbon; blue: molybdenum; scale bar, 50 nm) of porous MoCx nano-octahedrons.
A closer TEM examination on the edge of a MoCx nano-octahedron gives more details of the nanostructure. Figure 3d clearly shows that numerous MoCx clusters (darker area with visible lattice fringes indicated by green circles) are embedded in amorphous carbon matrix. Judging from their crystal lattices, the size of primary MoCx nanocrystallites is typically within 5 nm, although some seem to slightly aggregate and appear as larger clusters. The presence of amorphous carbon is also verified by thermogravimetric analysis and Raman spectrum (Supplementary Fig. 5), which might play important roles in prohibiting the growth of MoCx nanocrystallites and stabilizing the octahedral particles. A representative high-resolution (HR) TEM image (Fig. 3e) clearly shows lattice fringes with an interplanar distance of 0.24 nm, corresponding to the (006) planes of η-MoC. The uniform distribution of Mo and C elements is illustrated by EDX elemental mappings shown in Fig. 3f. Moreover, the MoCx nano-octahedrons exhibit a highly mesoporous structure, as evidenced by a high specific Brunauer–Emmett–Teller surface area of 147 m2 g−1 and abundant mesopores mainly distributed in the range of 4–10 nm (Supplementary Fig. 6). The mesopores in the MoCx nano-octahedrons are obviously larger than the pores in the pristine HKUST-1, which would be related to the substantial mass loss (that is, loss of C, H, O, Cu elements) during the carburization process. Such a porous structure with high uniformity is largely inherited from the ordered porous architecture of the MOFs precursor, which would benefit the application in electrocatalysis.
Electrocatalytic performance for HER
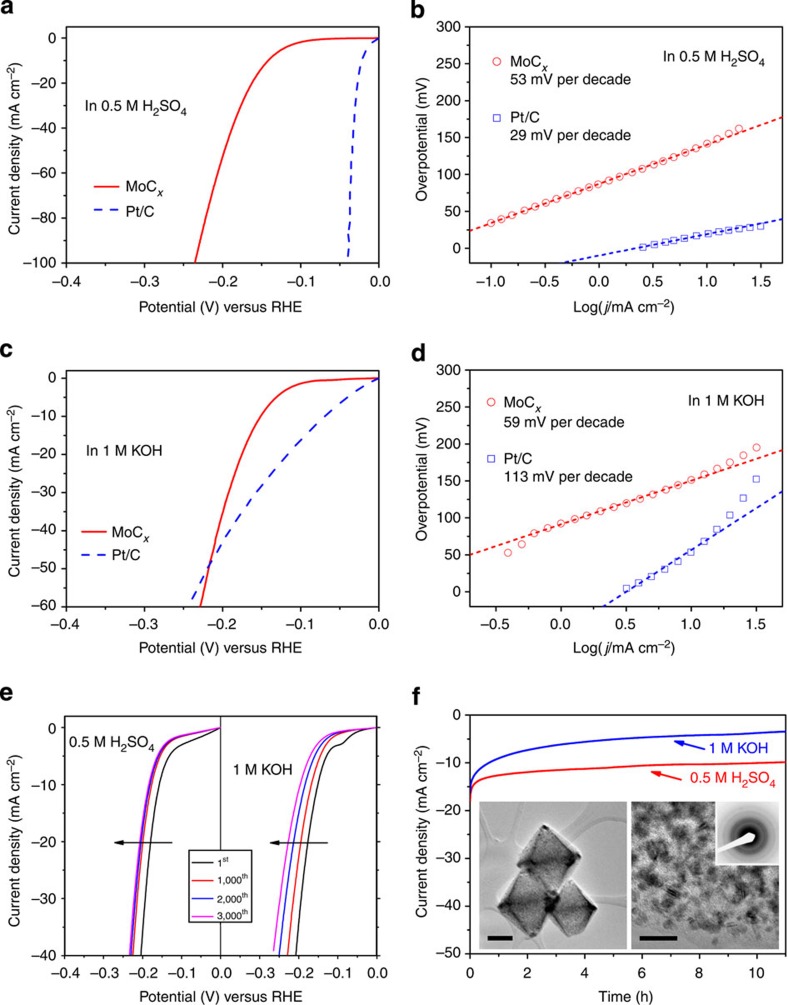
The as-prepared porous MoCx nano-octahedrons are evaluated as electrocatalysts for HER in both acidic and basic aqueous solutions. The porous MoCx nano-octahedrons exhibit optimal performance with a mass loading of 0.8 mg cm−2 on a glassy carbon (GC) disk electrode (Supplementary Fig. 7), while the catalysts prepared with shorter carburization time or at higher temperature show slightly inferior performance (Supplementary Fig. 8). The representative polarization curve (current density is based on geometric area of the electrode) and Tafel plot of the MoCx electrocatalyst in 0.5 M H2SO4 are shown in Fig. 4a,b, respectively, along with the performance of the benchmark Pt/C catalyst (40 wt% Pt on carbon black from Johnson Matthey, mass loading of 0.8 mg cm−2) for reference. As expected, the Pt/C catalyst exhibits excellent catalytic activity with an onset potential of ~0 V in acidic electrolyte. Meanwhile, the as-prepared MoCx electrocatalyst also shows a small onset potential of ~25 mV, estimated from the low current density region of the Tafel plot (Supplementary Fig. 9), beyond which the cathodic current increases rapidly48. To achieve current densities (j) of 1 and 10 mA cm−2, the MoCx electrocatalyst requires overpotentials (η) of ~87 and 142 mV, respectively. Tafel plots depicted in Fig. 4b show a small Tafel slope of 53 mV per decade for MoCx nano-octahedrons, higher than 29 mV per decade for the Pt/C catalyst. By extrapolating the Tafel plot, the exchange current density of MoCx nano-octahedrons can be calculated as 0.023 mA cm−2. Figure 4c,d shows the electrocatalytic properties of the MoCx nano-octahedrons and Pt/C in basic condition. Although the Pt/C catalyst exhibits a small onset potential close to 0 V compared with ~80 mV for the MoCx nano-octahedrons (Supplementary Fig. 10), the MoCx electrocatalyst outperforms the Pt/C catalyst for η≥220 mV with rapidly rising cathodic current. Small overpotentials of 92 and 151 mV are required for the MoCx nano-octahedrons to drive j=1 and 10 mA cm−2, respectively. In addition, the MoCx nano-octahedrons exhibit a smaller Tafel slope (59 mV per decade) than the Pt/C catalyst (113 mV per decade) as shown in Fig. 4d, along with an exchange current density of ~0.029 mA cm−2. The above comparison is based on the same loading mass of catalysts, which better reflects their performance in practical application and can be directly translated into their relative mass activity. Alternatively, we further compare the current density based on the mass of active materials (MoC for MoCx nano-octahedrons and Pt for Pt/C) and the turnover frequency assuming all metal atoms are involved in the HER process (Supplementary Fig. 11). Similar trends are observed. Specifically, the Pt/C catalyst possesses overwhelming advantage in acidic media, while in basic media the activity of MoCx nano-octahedrons approaches that of Pt/C catalyst at high overpotential. The electrochemical properties of the MoCx nano-octahedrons are summarized in Table 1, demonstrating the remarkable electrocatalytic HER activity in both acidic and basic solutions.
Figure 4. HER performance of porous MoCx nano-octahedrons.
(a) Polarization curve at 2 mV s−1 and (b) Tafel plots in 0.5 M H2SO4. (c) Polarization curve at 2 mV s−1 and (d) Tafel plots in 1 M KOH. (e) Polarization curves after continuous potential sweeps at 50 mV s−1 in 0.5 M H2SO4 (left) and 1 M KOH (right). (f) Time-dependent current density curves under η=170 mV in 0.5 M H2SO4 and η=180 mV in 1 M KOH (insets: TEM images and SAED pattern after 5,000 potential sweeps in 0.5 M H2SO4. Scale bars, left inset (200 nm) and right inset (20 nm)).
Table 1. Summary of HER activities of porous MoC x nano-octahedrons.
| Electrolyte | Onset η (mV versus RHE) | η at j=1 mA cm−2 (mV versus RHE) | η at j=10 mA cm−2 (mV versus RHE) | Tafel slope (mV per decade) | Exchange current density (mA cm−2) |
|---|---|---|---|---|---|
| 0.5 M H2SO4 | ~25 | 87 | 142 | 53 | 0.023 |
| 1 M KOH | ~80 | 92 | 151 | 59 | 0.029 |
HER, hydrogen evolution reaction.
To better understand the origin of such high electrocatalytic performance, we further compare our MoCx nano-octahedrons with the irregular MoCx nanoparticles (denoted as MoCx NPs, as shown in Supplementary Fig. 4 after grinding) as a reference sample, which exhibits similar composition but without well-defined nanostructure. Polarization curves in Supplementary Fig. 12 clearly reveal the much inferior performance of MoCx NPs with η≈230 mV to drive j=1 mA cm−2 in both acidic and basic solutions. To reveal whether the high activity comes from increased surface area, we compare the apparent electrochemical surface area (ECSA) of these two electrocatalysts by measuring the double-layer capacitance (Cdl), which is typically used to represent the ECSA (Supplementary Fig. 13)12,18. Surprisingly, the MoCx NPs actually possess quite similar Cdl, equivalent to ECSA, compared with MoCx nano-octahedrons. Although the high surface area of MoCx nano-octahedrons would obviously result in certain advantages when compared with bulky or low surface area materials, this is not the sole reason accounting for the high electrocatalytic activity.
Electrochemical impedance spectroscopy (EIS) analysis is performed on MoCx nano-octahedrons and MoCx NPs. Consistent with the previous studies, the EIS Nyquist plots of MoCx nano-octahedrons in both acidic and basic solutions exhibit two time constants (Supplementary Fig. 14). The first one at high frequency is related to the surface porosity of the electrode; the second one at low frequency, which depends on the overpotential, reflects the charge transfer process during HER25,27. Generally speaking, the charge-transfer resistance (Rct) shows strong correlation with the electrochemical performance. Thus, the Nyquist plots of MoCx nano-octahedrons and MoCx NPs at given overpotentials (that is, η=90 and 190 mV) in 0.5 M H2SO4 are compared and fitted to an equivalent electrical circuit with two time constants (Supplementary Fig. 15). It can be seen that for both samples the Rct substantially reduces at high overpotential. However, the value for MoCx nano-octahedrons is much smaller (about one order of magnitude lower) than that for MoCx NPs at the same overpotential, in line with their different HER activity. The small charge transfer resistance would be mainly related to the synergistic effect and strong interaction between the MoCx nanocrytallites and the continuous and in situ-incorporated carbon matrix, which ensures the facile electron transfer in the porous MoCx nano-octahedrons. Together with the above analysis of ECSA, we speculate that the high electrochemical activity of MoCx nano-octahedrons is due to their improved electronic/chemical properties and/or the exposure of more active sites, which are related to their unique mesoporous structure and small primary nanocrystallites. Nevertheless, more in-depth investigations would be necessary to reveal the detailed mechanism involved.
To assess the durability of the MoCx electrocatalyst, accelerated linear potential sweeps are conducted repeatedly on the electrodes at a scan rate of 50 mV s−1 as shown in Fig. 4e. In acidic condition, the polarization curves show a small shift of ~25 mV at j=10 mA cm−2 in the initial 1,000 sweeps, and then appear rather stable afterwards. On the other hand, the MoCx nano-octahedrons exhibit a continuous but small loss of activity in basic condition on repeated potential sweeps, implying some minor corrosion of electrocatalyst in the basic electrolyte. It should be noted that loss of electrocatalyst from the electrode on rapid rotation might also account for some degradation of the performance. The stability of the electrocatalyst is also evaluated by prolonged electrolysis at constant potentials, as shown in Fig. 4f. In line with the above studies, the current density of MoCx nano-octahedrons generally remains stable in 0.5 M H2SO4 for more than 10 h, whereas small degradation is observed in 1 M KOH on long-term operation. We have also examined the MoCx nano-octahedrons under TEM observation after continuous linear potential sweeps in acidic (insets of Fig. 4f) and basic (Supplementary Fig. 16) media. The nanostructure and crystallinity are well retained after the degradation measurement, again corroborating the good stability in acidic environment. However, some corrosion of the MoCx nano-octahedrons (especially the amorphous carbon) occurs during the potential sweeps in 1 M KOH. Such corrosion would cause the disintegration of the MoCx nano-octahedrons and loss of active materials, which is expected to account for the small but continuous degradation in basic condition.
Discussion
The MOFs-assisted strategy presented in this work is facile and easily reproducible to synthesize porous MoCx particles composed of a few nanometer-sized nanocrystallites. Compared with other synthesis methods, such as solid–gas phase reaction49 and pyrolysis of composites containing metal and carbon sources24,25, this MOFs-assisted approach guarantees homogeneous and efficient reaction, as well as uniform mesoporosity of the carbide product, originating from the unique crystalline structure with atomically hybridized MOFs matrix and Mo-based POMs. In addition, the highly localized and confined carburization process produces small nanocrystallites, which are embedded in an amorphous carbon matrix and prohibited from excessive growth. More importantly, the present strategy can be easily extended to synthesize tungsten carbide and molybdenum–tungsten mixed carbide (Supplementary Fig. 17), and potentially applicable to other early transition metals as well (Supplementary Note 1).
In virtue of the unique nanostructure, the porous MoCx nano-octahedrons exhibit excellent electrocatalytic activity for HER. In acidic aqueous electrolyte, the performance is among the best reported when compared with many representative noble metal-free electrocatalysts, such as various molybdenum-based compounds, transition metal dichalcogenides and phosphides (Supplementary Table 1). The HER performance in basic condition is also compared favourably with many HER catalysts (Supplementary Table 2). In particular, the electrocatalytic activity of MoCx nano-octahedrons in acidic media is comparable to the state-of-the-art β-Mo2C-based electrocatalysts with extra graphitic carbon supports (for example, graphene and/or carbon nanotubes)25,26,50. However, such high HER activity has not yet been achieved on other phases of molybdenum carbide41. Considering the sub-micrometre size of the MoCx nano-octahedrons, such catalytic activity is truly impressive. The high HER performance of our MoCx nano-octahedrons might be attributed to the following aspects. First, molybdenum carbides possess exceptional intrinsic HER activity, which is probably related to their Pt-like electronic and chemical properties22. Second, the small size of primary MoCx nanocrystallites and the high porosity render large electrochemical active surface and more importantly, perhaps more active sites to the MoCx nano-octahedrons. Third, the uniform morphology and mesoporous structure are expected to facilitate the charge and mass transport within these relatively large octahedral particles, thus promoting the hydrogen production process. Moreover, the amorphous carbon matrix might grant high robustness of the octahedral particles and provide extra protection for the ultrafine MoCx nanocrystallites with high surface energy. Nevertheless, the active materials in the centre part of MoCx nano-octahedrons might not be fully utilized. Thus, further improvement is highly expected by optimizing the size/porosity of the particles and/or incorporating supports (for example, carbon nanotubes, graphene sheets and integrated current collectors).
In summary, we report a novel MOFs-assisted strategy for synthesizing nanostructured MoCx nano-octahedrons as a highly active electrocatalyst for HER. This strategy relies on the confined and in situ carburization reaction occurring in a unique MOFs-based compound (NENU-5) consisting of a Cu-based MOFs (HKUST-1) host and guest Mo-based Keggin POMs resided in pores, which enables the uniform formation of metal carbide nanocrystallites without coalescence and excess growth. The as-prepared MoCx nano-octahedrons consist of ultrafine nanocrytallites with an unusual η-MoC phase embedded in an amorphous carbon matrix, and possess a uniform and highly mesoporous structure. Benefiting from the desirable nanostructure, these porous MoCx nano-octahedrons exhibit remarkable electrocatalytic activity for HER in both acidic and basic solutions with good stability. Moreover, such a strategy could be applicable for synthesizing other nanostructured transition metal carbides, thus opening new opportunities to develop high-performance functional materials for various applications.
Methods
Synthesis of NENU-5 nano-octahedrons
All chemicals were purchased and used without further purification. In a typical synthesis, 1 mmol of copper (II) acetate monohydrate (Sigma-Aldrich), 0.5 mmol of L-glutamic acid (Sigma-Aldrich) and 0.3 g of phosphomolybdic acid hydrate (Sigma-Aldrich) were dissolved in 40 ml of deionized water and stirred at ambient condition for 20 min. After that, 0.67 mmol of 1,3,5-benzenetricarboxylic acid (Merck) completely dissolved in 40 ml of ethanol was poured into the above solution under continuous stirring. The solution immediately turns turbid due to the rapid formation of NENU-5 nanocrystals. After stirring for 14 h at ambient condition, the green precipitate was collected by centrifugation and washed twice with ethanol. The product was dried at 70 °C overnight for further experiment and characterizations. The size of the NENU-5 particles can be easily tuned by varying the added amount of L-glutamic acid.
Synthesis of porous MoC x nano-octahedrons
The NENU-5 nano-octahedrons were heated in a tube furnace under N2 flow with a ramp rate of 2 °C min−1, maintained at 800 °C for 6 h and then cooled down naturally. The as-prepared sample was denoted as MoCx-Cu. The copper particles were removed by dispersing the sample in 0.1 M FeCl3 aqueous solution at ambient condition for 2 h. The resulting porous MoCx nano-octahedrons were collected by centrifugation, repeatedly washed with deionized water and then dried at 70 °C overnight.
Materials characterizations
The morphologies and structures of the products were characterized with FESEM (JEOL, JSM-6700 F, 5 kV) and TEM (JEOL, JEM-2010 and JEM-2100F, 200 kV). The chemical compositions and elemental mapping of the samples were analysed by EDX attached to JSM-7600F (FESEM, JEOL, 15 kV) and JEM-2100 F. The crystallographic information was collected by powder XRD (Bruker D8 Advance diffractometer with Cu Kα radiation λ=1.5406 Å). The N2 adsorption–desorption isotherms were collected using a Quantachrome Instruments Autosorb AS-6B at liquid-nitrogen temperature. Raman spectrum was collected using a Renishaw system 1000 micro-Raman spectroscope (Renishaw, UK). Thermogravimetric analysis was performed under air flow with a temperature ramp of 10 °C min−1.
Electrochemical measurements
The electrocatalytic activity was evaluated in a three-electrode configuration using a rotating disk electrode (PINE Research Instrumentation, at a rotation speed of 1500, r.p.m.) with an Autolab potentiostat/galvanostat (Model PGSTAT-72637) workstation at ambient temperature. A GC disk electrode (5 mm in diameter) served as the support for the working electrode. The catalyst suspension was prepared by dispersing 10 mg of catalyst in 2 ml of solution containing 1.9 ml of ethanol and 100 μl of 0.5 wt% Nafion solution followed by ultrasonication for 20 min. Then the catalyst suspension was pipetted using a micropipettor on the GC surface. The working electrode was dried at ambient temperature. A saturated calomel electrode (SCE) was used as the reference electrode and a graphite rod was used as the counter electrode. Potentials were referenced to a reversible hydrogen electrode (RHE): E(RHE)=E(SCE)+(0.242+0.059 pH)V. Linear sweep voltammetry was recorded in 0.5 M H2SO4 (pH=0.3) and 1 M KOH (pH=14) at a scan rate of 2 mV s−1 to obtain the polarization curves. The long-term stability tests were performed by continuous linear sweep voltammetry scans from −0.2 to −0.6 V (versus SCE, in 0.5 M H2SO4) and −1.0 to −1.4 V (versus SCE, in 1 M KOH) at a sweep rate of 50 mV s−1. All the data presented were corrected for iR losses and background current. To evaluate the ECSA, cyclic voltammograms were obtained from −0.2 to 0.2 V (versus SCE, in 0.5 M H2SO4) with sweep rates of 5, 10, 20, 50, 100 mV s−1. EIS was performed at various overpotentials with frequency from 0.1 to 100,000 Hz and an amplitude of 10 mV.
Author contributions
H.B.W. and X.W.L. conceived the idea and co-wrote the manuscript. H.B.W. carried out the synthesis. H.B.W. and B.Y.X. carried out the electrochemical evaluation. L.Y. & X.-Y.Y. helped in materials characterizations.
Additional information
How to cite this article: Wu, H. B. et al. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 6:6512 doi: 10.1038/ncomms7512 (2015).
Supplementary Material
Supplementary Figures 1-17, Supplementary Tables 1-2, Supplementary Note and Supplementary References.
Acknowledgments
We are grateful to the Ministry of Education (Singapore) for financial support through the AcRF Tier-1 funding (RG 12/13, M401120000).
References
- Lewis N. S. & Nocera D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010) . [DOI] [PubMed] [Google Scholar]
- Cook T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010) . [DOI] [PubMed] [Google Scholar]
- Gray H. B. Powering the planet with solar fuel. Nat. Chem. 1, 7–7 (2009) . [DOI] [PubMed] [Google Scholar]
- Liu Q. et al. Carbon nanotubes decorated with CoP nanocrystals: a highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 53, 5427–5430 (2014) . [DOI] [PubMed] [Google Scholar]
- Xie J. et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 135, 17881–17888 (2013) . [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl Acad. Sci. USA 110, 19701–19706 (2013) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. et al. Electrodeposited cobalt-sulfide catalyst for electrochemical and photoelectrochemical hydrogen generation from water. J. Am. Chem. Soc. 135, 17699–17702 (2013) . [DOI] [PubMed] [Google Scholar]
- Kibsgaard J., Chen Z., Reinecke B. N. & Jaramillo T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 11, 963–969 (2012) . [DOI] [PubMed] [Google Scholar]
- Voiry D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013) . [DOI] [PubMed] [Google Scholar]
- Popczun E. J., Read C. G., Roske C. W., Lewis N. S. & Schaak R. E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem. Int. Ed. 53, 5427–5430 (2014) . [DOI] [PubMed] [Google Scholar]
- Faber M. S. et al. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 136, 10053–10061 (2014) . [DOI] [PubMed] [Google Scholar]
- Vrubel H., Merki D. & Hu X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 5, 6136–6144 (2012) . [Google Scholar]
- Cao B., Veith G. M., Neuefeind J. C., Adzic R. R. & Khalifah P. G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 19186–19192 (2013) . [DOI] [PubMed] [Google Scholar]
- Li Y. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011) . [DOI] [PubMed] [Google Scholar]
- Gong M. et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 5, 4695 (2014) . [DOI] [PubMed] [Google Scholar]
- Popczun E. J. et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 9267–9270 (2013) . [DOI] [PubMed] [Google Scholar]
- Tian J., Liu Q., Cheng N., Asiri A. M. & Sun X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. Int. Ed. 53, 9577–9581 (2014) . [DOI] [PubMed] [Google Scholar]
- Hwu H. H. & Chen J. G. Surface chemistry of transition metal carbides. Chem. Rev. 105, 185–212 (2004) . [DOI] [PubMed] [Google Scholar]
- Oyama S. T. Preparation and catalytic properties of transition metal carbides and nitrides. Catal. Today 15, 179–200 (1992) . [Google Scholar]
- Michalsky R., Zhang Y.-J. & Peterson A. A. Trends in the hydrogen evolution activity of metal carbide catalysts. ACS Catal. 4, 1274–1278 (2014) . [Google Scholar]
- Chen W.-F., Muckerman J. T. & Fujita E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 49, 8896–8909 (2013) . [DOI] [PubMed] [Google Scholar]
- Vrubel H. & Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 51, 12703–12706 (2012) . [DOI] [PubMed] [Google Scholar]
- Chen W.-F. et al. Biomass-derived electrocatalytic composites for hydrogen evolution. Energy Environ. Sci. 6, 1818–1826 (2013) . [Google Scholar]
- Chen W. F. et al. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 6, 943–951 (2013) . [Google Scholar]
- Pan L. F. et al. Molybdenum carbide stabilized on graphene with high electrocatalytic activity for hydrogen evolution reaction. Chem. Commun. 50, 13135–13137 (2014) . [DOI] [PubMed] [Google Scholar]
- Liao L. et al. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 7, 387–392 (2014) . [Google Scholar]
- Furukawa H., Cordova K. E., O’Keeffe M. & Yaghi O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013) . [DOI] [PubMed] [Google Scholar]
- Kuppler R. J. et al. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 253, 3042–3066 (2009) . [Google Scholar]
- James S. L. Metal-organic frameworks. Chem. Soc. Rev. 32, 276–288 (2003) . [DOI] [PubMed] [Google Scholar]
- Torad N. L. et al. Direct synthesis of MOF-derived nanoporous carbon with magnetic Co nanoparticles toward efficient water treatment. Small 10, 2096–2107 (2014) . [DOI] [PubMed] [Google Scholar]
- Torad N. L. et al. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem. Commun. 49, 2521–2523 (2013) . [DOI] [PubMed] [Google Scholar]
- Jiang H.-L. et al. From metal–organic framework to nanoporous carbon: toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 133, 11854–11857 (2011) . [DOI] [PubMed] [Google Scholar]
- Yang S. J. et al. Preparation and exceptional lithium anodic performance of porous carbon-coated ZnO quantum dots derived from a metal–organic framework. J. Am. Chem. Soc. 135, 7394–7397 (2013) . [DOI] [PubMed] [Google Scholar]
- Xu X., Cao R., Jeong S. & Cho J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett. 12, 4988–4991 (2012) . [DOI] [PubMed] [Google Scholar]
- Chaikittisilp W. et al. Synthesis of nanoporous carbon–cobalt-oxide hybrid electrocatalysts by thermal conversion of metal–organic frameworks. Chem. Eur. J. 20, 4217–4221 (2014) . [DOI] [PubMed] [Google Scholar]
- Wu H. B. et al. Embedding sulfur in MOF-derived microporous carbon polyhedrons for lithium–sulfur batteries. Chem. Eur. J. 19, 10804–10808 (2013) . [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu H. B., Madhavi S., Hng H. H. & Lou X. W. Formation of Fe2O3 microboxes with hierarchical shell structures from metal–organic frameworks and their lithium storage properties. J. Am. Chem. Soc. 134, 17388–17391 (2012) . [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu H. B. & Lou X. W. Metal–organic-frameworks-derived general formation of hollow structures with high complexity. J. Am. Chem. Soc. 135, 10664–10672 (2013) . [DOI] [PubMed] [Google Scholar]
- Hou Y. et al. Metal−organic framework-derived nitrogen-doped core-shell-structured porous Fe/Fe3C@C nanoboxes supported on graphene sheets for efficient oxygen reduction reactions. Adv. Energy Mater. 4, 1400337 (2014) . [Google Scholar]
- Wan C., Regmi Y. N. & Leonard B. M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 53, 6407–6410 (2014) . [DOI] [PubMed] [Google Scholar]
- Sun C.-Y. et al. Highly stable crystalline catalysts based on a microporous metal−organic framework and polyoxometalates. J. Am. Chem. Soc. 131, 1883–1888 (2009) . [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Facile synthesis of a nanocrystalline metal–organic framework impregnated with a phosphovanadomolybdate and its remarkable catalytic performance in ultradeep oxidative desulfurization. ChemCatChem 5, 3086–3091 (2013) . [Google Scholar]
- Yan Z. et al. MoC-graphite composite as a Pt electrocatalyst support for highly active methanol oxidation and oxygen reduction reaction. J. Mater. Chem. A 2, 4014–4022 (2014) . [Google Scholar]
- Shohoji N. et al. Formation of hexagonal η-MoC1−x phase at a temperature lower than 1660 °C by solar radiation heating under presence of excess free carbon. Int. J. Refract. Met. H. Mater. 25, 220–225 (2007) . [Google Scholar]
- Hugosson H. W. et al. Theory of phase stabilities and bonding mechanisms in stoichiometric and substoichiometric molybdenum carbide. J. Appl. Phys. 86, 3758–3767 (1999) . [Google Scholar]
- Hall A. S., Kondo A., Maeda K. & Mallouk T. E. Microporous brookite-phase titania made by replication of a metal–organic framework. J. Am. Chem. Soc. 135, 16276–16279 (2013) . [DOI] [PubMed] [Google Scholar]
- Chen W.-F. et al. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012) . [DOI] [PubMed] [Google Scholar]
- Lee J. S., Oyama S. T. & Boudart M. Molybdenum carbide catalysts: I. Synthesis of unsupported powders. J. Catal. 106, 125–133 (1987) . [Google Scholar]
- Youn D. H. et al. Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube–graphene hybrid support. ACS Nano 8, 5164–5173 (2014) . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-17, Supplementary Tables 1-2, Supplementary Note and Supplementary References.