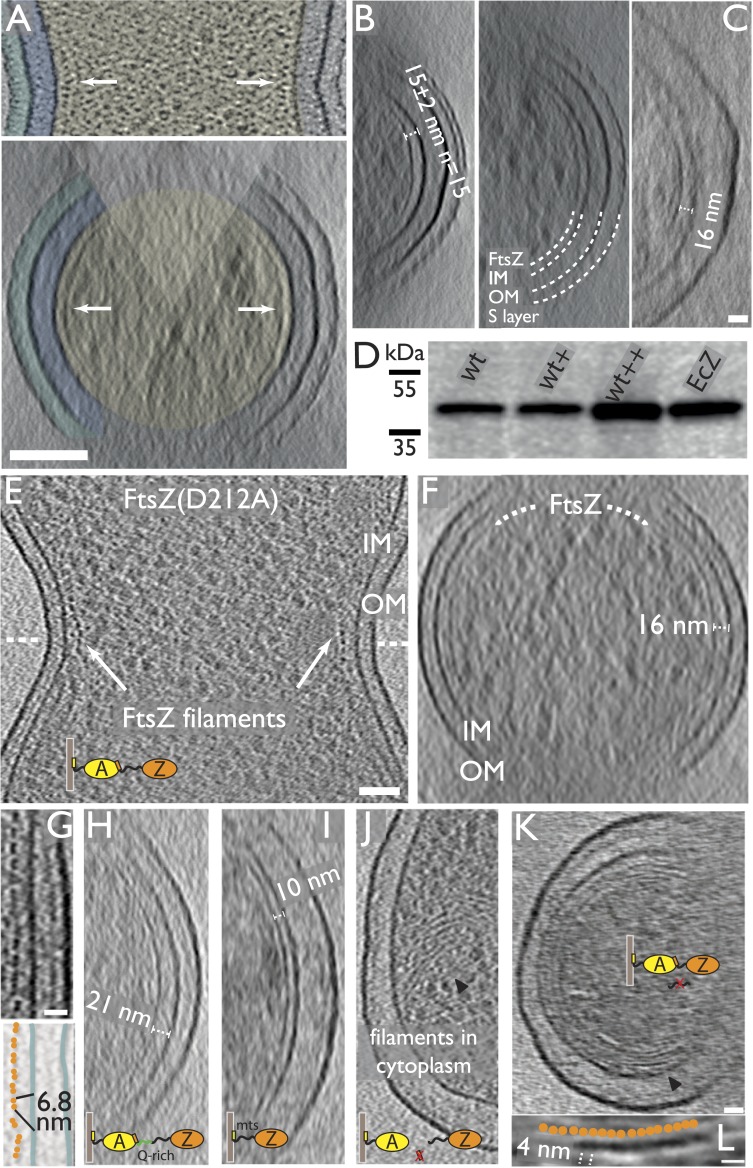
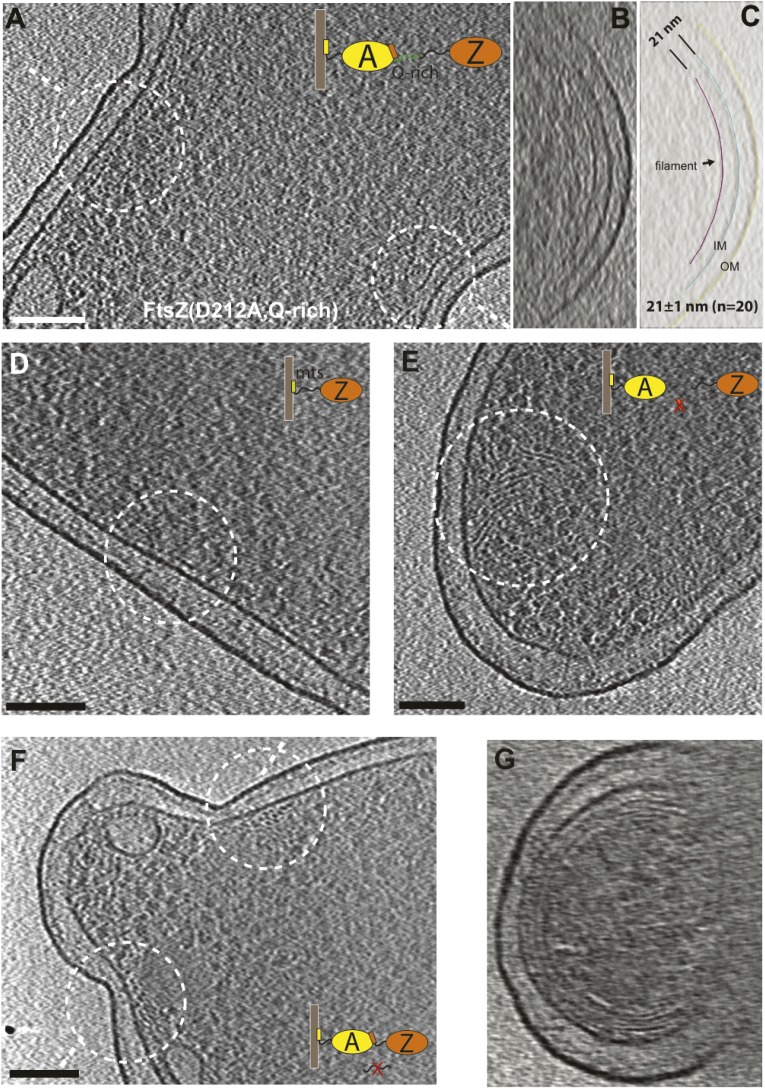
Figure 1. FtsZ forms bands of filaments completely encircling C. crescentus and E. coli division sites, as visualised by electron cryotomography.
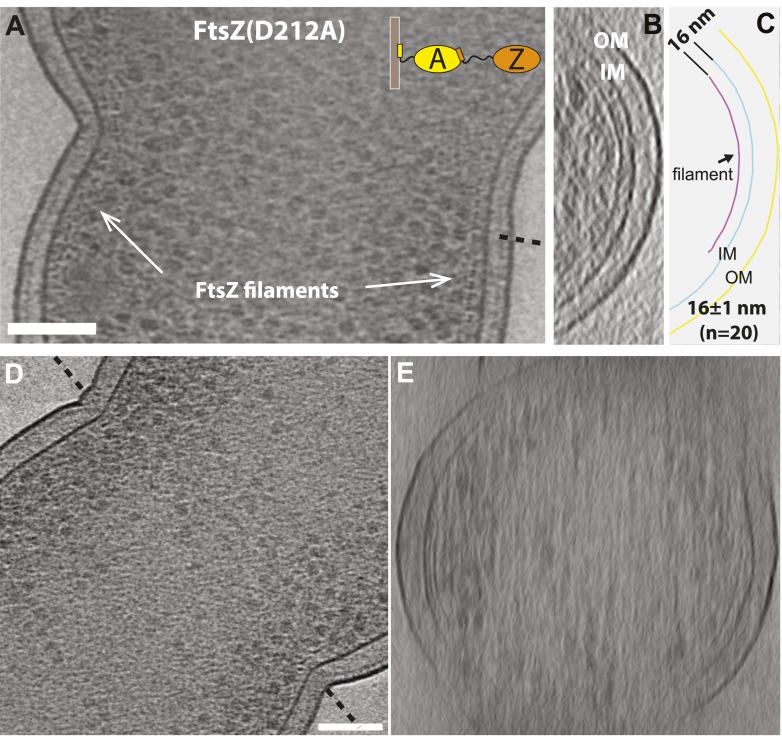
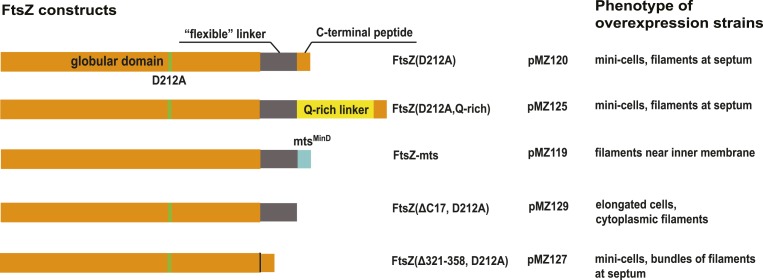
(A) C. crescentus NA1000/CB15N division site with filaments near the inner membrane IM (top panel, black dots highlighted by arrow, see also Video 1). Bottom panel shows the same cell rotated 90° around the short axis of the cell. The Z ring (arrow) is continuous and only invisible where there is no image because of the missing wedge (shaded triangle) (see Figure 1—figure supplement 1 for more details on the missing wedge problem). The cytoplasm (beige), periplasm (blue), and space between the OM and S layer (cyan) have been coloured for clarity. (B) More examples of continuous FtsZ rings found in C. crescentus cells. The filaments were on average 15 nm from the inner membrane. (C) Electron cryotomographic slice of the constriction site of a B/r H266 E. coli cell visualised perpendicular to the longitudinal axis, showing very similar FtsZ filaments when compared to C. crescentus (Figure 1A,B) and FtsZ(D212A) expressing E. coli cells (Figure 1F) and having roughly the same distance (16 nm) to the IM. Video 2 demonstrates the likely helical nature of the arrangement of the FtsZ filaments (see also Figure 1—figure supplement 2). (D) Western blot showing total FtsZ levels in cells used in (E–G) are about 2.5× that of wild-type cells. (+) refers to un-induced, (++) was induced by 0.02% arabinose. EcZ is purified E. coli FtsZ protein. (E–G) 10-nm thick electron cryotomographic slices of E. coli cells expressing FtsZ(D212A) protein in a wild-type B/r H266 background. See also Figure 1—figure supplement 3. (E) E. coli division site showing the cross-section of FtsZ filaments (single row of black dots) at the constriction site. See Video 3. (F) Visualisation of the same cell along the longitudinal axis shows that FtsZ filaments are located ∼16 nm from the inner membrane (IM). (G) Closer examination of the constriction site of another cell with higher expression level reveals FtsZ filaments form pairs, appearing as doublets of dark dots (upper) and orange spheres in the schematic illustration, on average 6.8 nm apart within the doublets (lower). (H–K) 10-nm thick electron cryotomographic slices of E. coli cells expressing engineered protein constructs based on FtsZ(D212A) (see also Figure 1—figure supplements 3,5 and Supplementary file 1, Table B). (H) Extending the C-terminal linker of FtsZ by inserting a linker sequence pushes the filaments further away from the IM (distance changed from 16 nm to a somewhat variable 16–21 nm). (I) Replacing the C-terminal FtsA-binding sequence of FtsZ with a membrane-targeting sequence (mts) makes FtsZ directly bind to the IM and results in FtsZ filaments closer to IM (distance changed from 16 nm to 10 nm). No cell constrictions were observed with this construct. (J) Removing the C-terminal FtsA-binding sequence of FtsZ renders it unable to maintain a fixed distance to the IM and FtsZ filaments that were observed within the cytoplasm. (K) Removing the C-terminal flexible linker of FtsZ makes it prone to form multiple layers of filaments that form complete rings or helices. Tomography using this construct works better because it produces small minicells. (L) A closer inspection of the area marked with the black arrowhead in G shows beads along the filament as illustrated by the schematic drawing with a repeat distance of 4 nm as expected for FtsZ filaments. IM: inner membrane; OM: outer membrane; WT: wild-type; Q-rich: FtsN-derived flexible linker; mts: membrane-targeting sequence. Scale bars: 100 nm in (A) and (B), 50 nm in (E, F, H, I, J), 20 nm in (C, G, K), 10 nm in (H), 20 nm in (L).