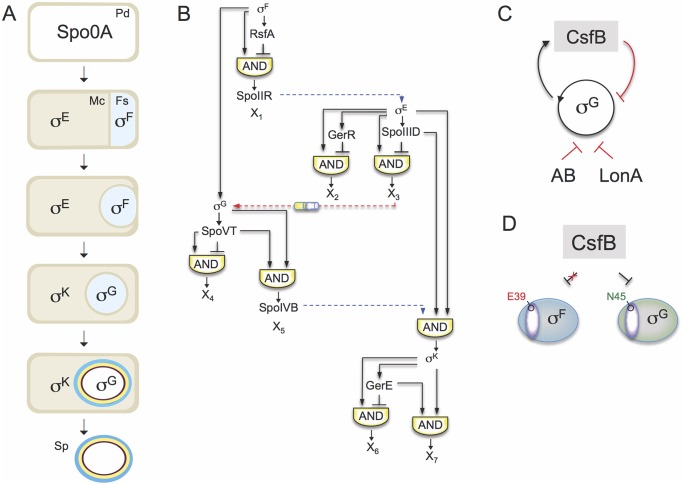
Fig 1. The sporulation network and the action of CsfB on σG.
A: the main morphological stages of sporulation are represented, with the main regulatory proteins active in the indicated cells. Pd, pre-divisional cell; Mc, mother cell; Fs, forespore; Sp, mature spore. B: organization of the transcriptional network of sporulation. The broken blue lines represent cell-cell signalling pathways. First, σF drives production of a protein, SpoIIR, secreted to the intermembrane space. SpoIIR then activates a membrane-embedded protease that triggers the proteolytic activation of pro-σE [18]. σE and σF activity are required for the assembly of a cell-cell secretion system (the SpoIIQ-SpoIIIAH channel in the figure) that, following engulfment completion, allows the mother cell to nourish the isolated forespore, thus enabling continued macromoelcualr synthesis and activation of the σG auto-regulatory loop [67]. Lastly, σG controls production of a signaling protein, SpoIVB, secreted into the intermembrane space that activates the machinery responsible for the proteolytical activation of σK [18]. The negative feedback loop through which σK limits production of σE is omitted for simplicity. C: the panel represents the composite negative feedback loop that operates in pre-divisional cells, and possibly also in the forespore prior to engulfment completion, to prevent activation of the σG positive auto-regulatory loop. Transcriptional and protein-protein interactions are shown in black and red, respectively. D: a single amino acid residue in region 2.1 (purple sector) allows CsfB to discriminate between the highly similar forespore sigma factors σF and σG: N45 of B. subtilis σG allows binding by CsfB, whereas a glutamate at the same position precludes binding. Conversely, a glutamate at the homologous position of σF (E39) impedes binding by CsfB whereas an asparagine at the same position is sufficient for binding. N45 and E39 are conserved among Bacillus orthologues of σG and σF.