Abstract
Prenatal exposure to ethanol results in sensory deficits and altered social interactions in animal and clinical populations. Sensory stimuli serve as important cues and shape sensory development; developmental exposure to ethanol or sensory impoverishment can impair somatosensory development, but their combined effects on behavioral outcomes are unknown. We hypothesized that 1) chronic prenatal ethanol exposure would disrupt social interaction and somatosensory performance during adolescence, 2) that a mild sensory impoverishment (neonatal unilateral whisker clipping; WC) would have a mildly impairing to sub-threshold effect on these behavioral outcomes, and 3) that the effect of ethanol would be exacerbated by WC. Long-Evans dams were fed a liquid diet containing ethanol or pair-fed with non-ethanol diet on gestational day (G) 6-G21. Chow-fed control animals were also included. One male and female pup per litter underwent WC on postnatal day (P)1, P3, and P5. Controls were unclipped. Offspring underwent social interaction on P28 or P42, and gap-crossing (GC) on P31 or P42. Ethanol-exposed pups played less and crossed shorter gaps than control pups regardless of age or sex. WC further exacerbated ethanol-induced play fighting and GC deficits in all males but only in 28-day-old females. WC alone reduced sniffing in all males and in younger females. Thus, prenatal ethanol exposure induced deficits in social interaction and somatosensory performance during adolescence. Sensory impoverishment exacerbates ethanol's effect in 28-day-old male and female animals and in 42-day-old males, suggesting sex-and age-dependent changes in outcomes in ethanol-exposed offspring.
Keywords: fetal alcohol spectrum disorder, barrel cortex, play behavior, sensory impoverishment, gap crossing, rat
1. INTRODUCTION
Developmental exposure to ethanol results in sensory deficits and altered social interaction in humans and in animal models [1-13]. The range and severity of these outcomes are sensitive to several factors (including timing, dose, and duration of ethanol exposure, as well as maternal genetics, nutritional status, and age [14-19]). In humans, social deficits can range from minimal to severe depending on such factors, but can manifest as poor social skills, difficulties understanding social cues, and inappropriate social behavior that causes problems within the home, at school, or with the law [7, 13, 20, 21]. Also apparent in this population are sensory deficits including sensory processing deficits and problems transferring somatosensory information between hemispheres [1, 11, 13, 21].
Ethanol-induced alterations in social behavior have been described in rodent models; for example, switched sexual dimorphic social play patterns in adolescents [4, 22], delayed maternal responses in dams that were prenatally exposed, increased aggression in prenatally exposed adult males [10, 23], and altered sexual interactions in both sexes [24, 25]. In addition to altered social behavior, sensory detection and processing of somatosensory stimuli are also impaired following prenatal ethanol exposure [2, 6]. This may be due, in part, to the ethanol-induced alteration in the structure of somatosensory cortex [5, 26-30].
Sensory stimuli play an important role in both cueing and shaping social interaction in rodents [31-35]. Damage to or removal of the somatosensory cortex alters play behavior [31]. Similarly, numbing of the animals nape (thereby decreasing sensation and inhibiting activity in the somatosensory system) reduces play interaction [2, 33].
Disrupting input into the somatosensory system during development also alters its structure and somatosensory-dependent behaviors. This can be modeled by damaging whisker follicles or clipping whiskers during the first postnatal week when thalamocortical afferents develop the typical barrel pattern [36, 37]. These manipulations cause a sensory impoverishment that has long-term effects on cortical morphology and function of the somatosensory system, including increased neuronal activity and increased size of the excitatory receptive field of the rodent barrel cortex [38-41]. Impoverishment can also have a long-term effect on behavior; bilateral neonatal whisker clipping the first three days of life leading to decreased performance on a somatosensory-dependent task (gap crossing) and increased social interaction during play with no effect on anxiety as measured by elevated plus maze performance [38].
Chronic prenatal exposure to ethanol alters the structure of the somatosensory system; reducing neuronal numbers in trigeminal nuclei [28] and the somatosensory cortex [5], and stunting thalamocortical afferents to cortex [42]. There is also a delay in the emergence of barrels in the somatosensory cortex and lower neuronal density within barrels [26, 27, 29, 30]. Depressed neuronal activity is seen in the adult somatosensory cortex of ethanol-exposed animals [43-45] and delays in cellular maturation, such as expression of N-methyl-D-aspartate receptor isoforms, have also been reported following prenatal ethanol exposure [6, 46]. Taken together this shows that prenatal ethanol exposure alters the structure and function of this system.
While both prenatal ethanol exposure and sensory impoverishment have distinct effects on the developing brain and consequent behavior, only one study has investigated the effect of disruption of somatosensory function and developmental ethanol exposure in concert on social behavioral outcomes [2]. In this study, rats were exposed to ethanol from gestational day (G)1 to G22 and postnatal day (P)2 to P10 and underwent a social interaction test during adolescence. Ethanol-exposed rats showed increased pinning during social play. Acute inhibition of tactile (somatosensory) input by numbing the nape depressed pinning in control animals at the highest dose of xylocaine tested. Ethanol-exposed animals that were numbed demonstrated increased pinning and were more sensitive to degrading touch cues, showing reductions in pinning at lower xylocaine doses during social interaction in comparison to controls. This suggests ethanol-induced difficulties in processing somatosensory cues alter social behaviors and that acute disruptions to sensory stimuli can directly exacerbate the ethanol effect.
The current study tested the hypotheses (1) that prenatal ethanol exposure disrupts social interaction, elevated plus maze, and whisker-related somatosensory performance (gap crossing) during adolescence, (2) that a modest sensory impoverishment during early development (neonatal unilateral whisker clipping; WC) would have mild but long-term effects on social interaction and gap crossing, and (3) that the double-hit of prenatal ethanol exposure and WC would have the greatest effect on the behaviors tested.
2. METHODS
2.1 Subjects
Timed pregnant Long Evans dams (Harlan, Fredrick, MD, USA) were brought in on G3 (G1 was designated as the first day a sperm-plug was identified). Animals were housed in an AAALAC -approved facility at the University Of Maryland, Baltimore that is humidity- (40-45%) and temperature-controlled (22°C) and maintained on a 12/12-hr light/dark cycle (lights on at 0700). All procedures were performed with approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland, Baltimore and were in accordance with the guidelines for animal care established by the National Institutes of Health.
2.2 Prenatal exposure
Starting on G6, dams were randomly assigned to one of three prenatal exposure conditions. Two groups received a liquid diet (Bio-serve, Frenchtown, NJ). One group (ET) received diet containing 11.5% ethanol-derived calories (EDC) on G6 and G7, 22% EDC on G8-G10, and 35% EDC between G11 and G20. Blood ethanol concentrations typically reach 100-150 mg/dl [47, 48]. A second group (PF) was pair fed an isonutritive, isocaloric liquid diet containing maltose in place of the ethanol. The third group (CH) received ad libitum access to laboratory chow. All animals had ad libitum access to water. On G21, liquid diet-fed dams were returned to regular chow. Birth typically occurred on G22 (also designated as P0).
2.3 Postnatal manipulation
Within 24 hr of birth, all litters were surrogate fostered to a CH dam. Litter information was recorded (litter size, sex ratio, average male and female pup weights) on P1, then litters were culled to 10 keeping the ratio of males to females at 6:4 as best as possible. One male and one female pup from each litter were randomly assigned to a postnatal unilateral whisker clip (WC) group; on P1, P3, and P5, the right whiskers were clipped 1mm or less from the skin using a sharp surgical scissors. One male and one female pup from each litter was left unclipped and assigned to the non-whisker clipped (NWC) group. Pups were weaned on P21 and housed in same sex littermate pairs.
A total of 54 litters were used to generate 225 male and female offspring to serve as experimental subjects. An additional 32 untreated litters provided the 225 social interaction partners. To avoid potential litter bias no more than one male and one female from a litter was allotted to each treatment condition per testing age [49]. Animals were divided into two distinct cohorts. One cohort underwent social interaction (SI) testing at P28, followed by gap crossing at P31 and elevated plus maze testing at P35; a second cohort of animals underwent SI testing and gap crossing at P42.
2.4 Measurement of whisker length
On P42, a sub-set of 70 rats were anesthetized with 3% isoflurane (Vet One, Boise, ID) until unconscious, and C-row whiskers (C1 to C5) were plucked from the base of the muscle on both the clipped and non-clipped sides. The length (mm) for each whisker from the base to the tip of the whisker shaft was measured and recorded.
2.5 Social Interaction Test
On P28 or P42 rats were transported to a dimly lit testing room where they habituated to the room for 2 h before undergoing testing in a modified social interaction (SI) paradigm [9, 50, 51]. The SI apparatus was a perspex box (30 cm × 20 cm × 20 cm; Binghamton Plate Glass, Binghamton, NY). This box included a clear partition that divided it into two equal halves with a semicircular hole (7 cm × 5 cm) in the middle, which allowed the animals to temporarily avoid contact by moving between compartments [9, 50]. Each test comprised a 10 min interaction between an experimental animal and an untreated play partner. Animals were matched for weight (weight difference no greater than ±10 g), age, and sex.
All animals underwent a 30 min isolation; play partners spent the entire 30 min isolation in a holding cage. Experimental subjects had their sides marked with a non-toxic red Sharpie™ marker and then spent 20 min in a holding cage and 10 min in the SI box. During this habituation to the SI box, locomotor activity was tracked and recorded using a Sentech STC-TB83USB-AS camera and Anymaze™ software (San Diego Instruments, San Diego, CA). Locomotor activity dependent measures included: total distance traveled (m) and average speed of travel (m/s).
Following habituation, a play partner was introduced into the SI box and animals were recorded for 10 min (using the camera and AnyMaze™ software as above). Videos were scored by a trained observer blinded to experimental conditions. SI behaviors scored included: 1. play fighting, which included tags (forelimb or muzzle contacts to the nape area) and pins (when the experimental animal rolled the play partner onto it's back), 2. social motivation (social motivation is defined as a coefficient (%) = [(crossing the partition towards the play partner – crossing the partition to get away from the partner)/ total partition crosses]) 3. chasing (time spent in pursuit/following the play partner), and 4. sniffing (time spent sniffing the play partner)[52].
The SI box was wiped with towels dampened with a dilute cleaning solution containing 0.26%. alkyl dimethyl benzyl ammonium chloride between subjects to dislodge any confounding scents remaining in the box.
2.6 Gap Crossing
Somatosensory performance was examined using a modified gap-crossing task [38, 39] on P31 or P42. Rats were placed on a drawbridge (15 cm wide × 60 cm long) 31 cm above the floor of a well-lit white box (78 × 48 × 62 cm) and traversed an increasing gap to a dark escape box (25 × 25 × 25 cm). Initial starting distance to the escape box was 1 cm and was increased by 1 cm after each successful trial until they failed cross the same distance twice in a row, either by falling or by refusing to cross within the 120s trial time limit. As only a total of 14 falls from 12 subjects was recorded throughout the study, the distance of the longest gap successfully crossed was recorded as the dependent meassure.
2.7 Elevated plus maze
On P35, anxiety was assessed in the first cohort of animals using an elevated plus maze (Stoelting, Wood Dale, IL). Animals were placed in the center of the plus, and activity was tracked for 10 min using the Anymaze™ software and camera system described above. Measures recorded included: total distance traveled, speed, and preference for the open arms: [(time (s) in open arm - time in closed arm)/total time].
2.8 Experimental Design and Data Analysis
For each measure, mean and standard error of the mean (SEM) was generated. An initial 4-way analysis of variance (ANOVA) was performed with each social behavior metric to assess sex interactions. Because there are innate sex differences in rat social behavior [53] as well as sex-dependent effects of ethanol [4, 54-57], follow-up 3-way ANOVAs were performed on data sets that were separated by sex. Study design for social interaction and gap crossing tests was a 3 (prenatal exposure: ET, PF, or CH) × 2 (postnatal manipulation: WC or NWC) × 2 (age: P28 or P42) × 2 (sex: male or female) factorial. Elevated plus maze data was only collected at one age, thus the design for this behavior was a 3 (prenatal) × 2 (postnatal) × 2 (sex) factorial. All dependent measures were assessed using separate between group analyses of variance (ANOVAs); Tukey's post hoc comparisons were used to determine group differences in both the main and interactive effects. All non-behavioral data related to dam and litter information were assessed using one-way ANOVA or one-way ANOVA on ranks as applicable. Whisker lengths were assessed using a three-way ANOVA with sex serving as the third variable. The α for all statistical comparisons was set as p<0.05.
Greater sensory processing difficulties have been well documented in ethanol exposed offspring [1,2,11] compared to non-exposed offspring, and work by Charles-Lawerence et al [2] shows greater sensitivity in these ethanol exposed offspring to acute sensory impoverishment can effect social behavior. This prompted the direct a priori comparisons between ET/NWC and CH/NWC groups as well as between ET/WC and ET/NWC groups regardless of the presence of prenatal by postnatal exposure interaction, to detect the effect neonatal sensory impoverishment can have on social behavior. This should serve to clarify the data by subverting type 2 error common in studies that have a large number of experimental factors and relatively subtle effect sizes. To correct for family-wise error that arises with multiple planned comparisons, Bonferroni corrections were applied.
3. RESULTS
3.1 Dam and Litter Data
Dams were weighed on G6, G13, and G20 and their average daily weight gain was calculated for all prenatal treatment groups. Assessment of maternal weight gain revealed no significant differences among the groups (Table 1). Nor were dam weights different just prior to birth (mean ± SEM: CH, 320 ± 6.81 g, PF, 321 ± 4.82 g, ET, 312 ± 6.14 g).
Table 1.
Mean dam and litter outcomes ± S.E.M.
| Prenatal | # dams | Dam wt gain (g/day) | Litter ratio (% male) | Prenatal exposure |
|---|---|---|---|---|
| CH | 17 | 6.21 ± 0.42 | 45.21 ± 3.57 | 5.91 ± 0.14 |
| PF | 18 | 7.11 ± 0.37 | 47.70 ± 3.97 | 5.89 ± 0.18 |
| ET | 19 | 6.27 ± 0.29 | 51.47 ± 4.98 | 5.63 ± 0.16 |
There was no effect of prenatal exposure on litter size, litter sex ratio, or average pup body weights on P1 (Table 1). Litter size averaged ~9 pups per litter, ranging from 6 to 15 pups. Overall, ~48% of pups were male. Pup weights on P1 ranged from 4.8 g to 7.6 g, but averaged 5.7 g overall.
3.2 Whisker length outcomes
Three-way ANOVA analysis revealed no effect of whisker clipping or sex on the length of the majority of the C-row whiskers measured on P42. There was however a main effect of prenatal exposure on the C1 whisker (F2,58 = 3.855, p=0.027); Et exposed offspring had slightly longer (~0.02 mm) C1 whiskers than the CH-exposed cohort (p=0.02). Prenatal exposure had no effect on the other C-row whisker lengths (Table 2).
Table 2.
Mean group whisker lengths (mm) ± S.E.M.
| Prenatal exposure | Sex | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|
| CH | ||||||
| NWC | M | 1.10 ± 0.05 | 1.70 ± 0.11 | 2.64 ± 0.10 | 3.96 ± 0.14 | 4.82 ± 0.24 |
| WC | M | 1.25 ± 0.09 | 2.05 ± 0.10 | 2.83 ± 0.13 | 3.65 ± 0.15 | 4.68 ± 0.01 |
| PF | ||||||
| NWC | M | 1.24 ± 0.05 | 2.01 ± 0.15 | 2.93 ± 0.16 | 3.87 ± 0.18 | 4.62 ± 0.24 |
| WC | M | 1.31 ± 0.11 | 2.02 ± 0.10 | 3.02 ± 0.09 | 4.03 ± 0.18 | 5.02 ± 0.22 |
| ET | ||||||
| NWC | M | 1.33 ± 0.09* | 1.95 ± 0.16 | 3.07 ± 0.12 | 3.77 ± 0.21 | 4.82 ± 0.14 |
| WC | M | 1.37 ± 0.07* | 1.98 ± 0.04 | 2.78 ± 0.14 | 3.70 ± 0.10 | 4.72 ± 0.06 |
| CH | ||||||
| NWC | F | 1.10 ± 0.07 | 1.82 ± 0.16 | 2.78 ± 0.05 | 3.94 ± 0.14 | 4.78 ± 0.22 |
| WC | F | 1.22 ± 0.10 | 1.98 ± 0.11 | 2.79 ± 0.14 | 3.67 ± 0.19 | 4.62 ± 0.10 |
| PF | ||||||
| NWC | F | 1.24 ± 0.06 | 1.98 ± 0.13 | 3.00 ± 0.18 | 3.96 ± 0.20 | 4.56 ± 0.23 |
| WC | F | 1.30 ± 0.10 | 1.98 ± 0.11 | 3.08 ± 0.11 | 4.14 ± 0.19 | 5.04 ± 0.17 |
| ET | ||||||
| NWC | F | 1.28 ± 0.07* | 1.97 ± 0.16 | 3.10 ± 0.11 | 3.80 ± 0.22 | 4.78 ± 0.13 |
| WC | F | 1.36 ± 0.05* | 1.97 ± 0.04 | 2.80 ± 0.13 | 3.72 ± 0.11 | 4.68 ± 0.07 |
The C1 whiskers of rats prenatally exposed to ethanol were longer than those of CH-fed offspring
p < 0.05.
3.3 Social interaction (SI)
3.3.1 Play fighting
Assessment of play fighting using a 4-way ANOVA showed significant prenatal exposure by sex (F 2,206 = 4.50, p=0.01) and sex by age interactions (F 1,206 = 6.25, p=0.01) (Figure 1A). Post hoc revealed that male offspring of PF- and CH-fed dams play fought with their play partners more than female offspring(p<0.001 and p=0.008, respectively). This sex effect was absent in the ET rats: prenatal ethanol exposure reduced play fighting in males to levels comparable to that of their female counterparts. There was also an age effect present in the females that was absent in the males, with the 28-day-old females play fighting more than their older counterparts (p=0.001). The data set was then separated by sex for further assessment.
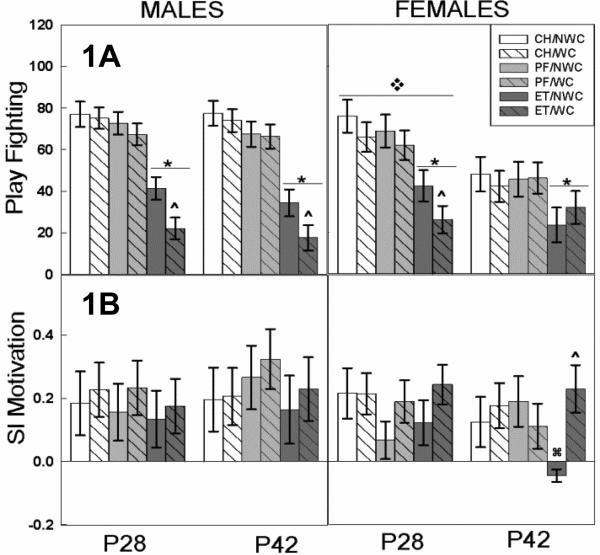
Figure 1. Social Interaction (SI): Play Fighting and SI Motivation.
Effects of ET and WC on play fighting and SI motivation in male (left) and female (right) offspring tested during pre-adolescence (P28) or mid-adolescence (P42). Bars depict group mean and T bars the standard error of the mean. N ranged from 8-12 per sex per treatment group.
A) ET groups showed reduced play fighting compared to PF or CH groups regardless of age or sex ( p<0.05). ET/WC males played less than ET/NWC males (^ p<0.05). At P28 ET/WC females also showed decreased play compared to ET/NWC females (^ p<0.05). Overall, females engaged in more play at P28 than at P42 (
p<0.05). ET/WC males played less than ET/NWC males (^ p<0.05). At P28 ET/WC females also showed decreased play compared to ET/NWC females (^ p<0.05). Overall, females engaged in more play at P28 than at P42 ( p<0.05).
p<0.05).
B) The ET/NWC female were less motivated to engage in social interaction at P42 than either the CH/NWC ( p<0.05) or the ET/WC females (^ p<0.05).
p<0.05) or the ET/WC females (^ p<0.05).
3.3.2 Play Fighting (males)
Play fighting showed a significant interaction of prenatal exposure by postnatal manipulation (F 2,99 = 3.16, p=0.04). Both PF and CH rats play fought with their play partner actively (~60-80 times per 10 min play period) during the session. In comparison, post hoc analysis revealed rats prenatally exposed to ethanol showed decreased play fighting, playing with their play partner half as much as either the CH or PF exposed rats (p<0.001 for both groups). Whisker clipping further exacerbated the ethanol-induced play fighting deficits (p=0.006) (Figure 1A left). Play fighting was unaffected by the age of testing.
3.3.3 Play fighting (females)
Examination of play fighting in females revealed significant main effects of age (F 1,104 = 13.03, p<0.001) and prenatal exposure (F 2,104 = 12.96, p<0.001; Figure 1A right). Female rats engaged in more play fighting at P28 than at P42 (p<0.001). Prenatal exposure to ethanol decreased the amount of play fighting at both ages compared to both CH and PF exposed controls (p< 0.001 for both). There was no significant main effect or interaction of whisker clipping on female play fighting behavior in the initial ANOVA assessment, however, a priori assessment of the 28 day-old ET-treated animals showed that ET/WC pups play fought less than the ET/NWC group (t 21 = 1.92, p=0.03).
3.3.4 Social Motivation (males)
The 4-way ANOVA showed that sex did not interact with any between group factors, however there was a trend for males to be more socially motivated than females (p=0.06). The data was split by sex for further assessment. In males, all groups showed a positive preference, crossing more often to be in the same chamber as the play partner than crossing away (Figure 1B left). There were no significant effects of prenatal exposure or postnatal manipulation.
3.3.5 Social Motivation (females)
In females, social motivation outcomes demonstrated no significant effect of any of the between group factors in the initial ANOVA. Most groups showed a positive preference, crossing more often toward the play partner than away; the exception was the 42-day-old ET/NWC animals which showed a negative coefficient (Figure 1B). At P42, a priori comparisons between ET/WC vs CH/NWC (t 14=1.743, p=0.05) and ET/WC vs ET/NWC (t15 =−2.64, p=0.04) groups demonstrated the ET/NWC to have a significant social avoidance (Figure 1B right).
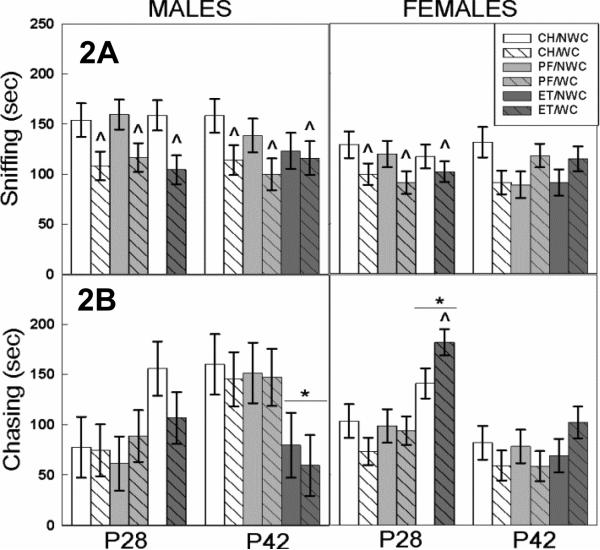
3.3.6 Sniffing (males)
The 4-way ANOVA revealed a postnatal manipulation by sex by age effect (F 1,206 = 5.92, p=0.02) on sniffing behavior. Although time spent sniffing the play partner was not affected by prenatal exposure or the age at which the animal was tested in males, postnatal manipulation significantly reduced sniffing in WC males compared with NWC males (F 1,99 = 17.34, p<0.001), regardless of age or prenatal exposure (Figure 2A left).
Figure 2. SI: Sniffing and Chasing.
Effects of ET and WC on sniffing and chasing in male (left) and female (right) offspring tested during pre-adolescence (P28) or mid-adolescence (P42). Bars depict group mean and T bars the standard error of the mean. N ranged from 8-12 per sex per treatment group.
A) At both ages, WC males sniffed less than NWC males (^ p<0.05). 28-day-old WC females also showed decreased sniffing compared to NWC females at the same age (^ p<0.05).
B) ET males chased their partners less at P42 than either the CH or PF males ( p<0.05). In contrast, the 28-day-old ET females chased their partners more than CH or PF controls (
p<0.05). In contrast, the 28-day-old ET females chased their partners more than CH or PF controls ( p<0.05); ET/WC females chased more than the ET/NWC females (^ p<0.05).
p<0.05); ET/WC females chased more than the ET/NWC females (^ p<0.05).
3.3.7 Sniffing (females)
In females, sniffing showed an interaction of age and postnatal manipulation (F 2,104 = 4.11, p=0.02; Figure 2A right). Whisker clipping significantly (p= 0.006) decreased time spent sniffing by 28-day-old animals, but did not affect this behavior at P42.
3.3.8 Chasing (males)
The 4-way ANOVA assessment revealed significant prenatal exposure by sex and age by sex interactions (F 2,206 = 4.00, p=0.02 and F 1,206 = 14.40, p<0.001, respectively) as well as a trend for a prenatal exposure by postnatal manipulation by sex interaction (p=.06). Post hoc analysis revealed that CH and PF males chased more than females (p=0.02, for both groups) and that this effect of sex was absent in ET animals and that overall 42-day old males chased more than females this sex effect was not seen in the younger animals. The data set was then split by sex for further assessment.
In males, there was a significant age × prenatal exposure interaction (F 2,99 =7.63, p<0.001) on male chasing during play. Prenatal ethanol exposure significantly reduced time spent in pursuit of the play partner compared to both the CH (p=0.018) and PF (p=0.027) animals at P42, but not at P28. There was no difference in chasing between the CH and PF groups. The pattern of chasing was significantly altered between prenatal treatment groups at the different ages; CH and PF animals chased less on P28 and more on P42 (p=0.008 and p=0.009, respectively), whereas ET pups chased more on P28 and less on P42 (p=0.035; Figure 2B left).
3.3.9 Chasing (females)
In females, time spent chasing showed a significant age by prenatal exposure interaction (F 1,104 = 4.09, p=0.045; Figure 2B right). Breakdown of this revealed that prenatal ethanol exposure increased chasing behavior compared to both the CH and PF controls in the 28- but not the 42-day-old animals. PF and CH rats spent a similar amount of time chasing their play partners at both ages.
There was also a significant prenatal exposure × postnatal manipulation interaction (F 1,104 = 4.71, p=0.03) on female chasing. Post hoc examination of data collapsed across age revealed that the ET/WC animals chased more than all other treatment groups (p<0.020 for all groups); however, assessment of the ages separately using a two-way ANOVA showed this effect to be driven by whisker clipping exacerbating chasing in the 28-day-old ET females (p<0.001). No significant effect of whisker clipping was found in either the PF or CH control groups.
3.4 Locomotor activity
3.4.1 Locomotor activity during habituation (males)
While the 4-way ANOVA did not detect a significant main effect or interaction containing sex, there was a trend for a prenatal exposure by postnatal manipulation by sex interaction (p=0.08) thus the data for males and females were assessed separately. In males, locomotor activity during habituation to the SI apparatus was not altered by prenatal exposure or postnatal manipulation, but did differ as a function of age.. A main effect of age was noted for distance traveled (F 1,99 = 9.648, p=0.002) and speed (F 1,99 = 7.98, p=0.006; Table 3). Animals tested on P42 were significantly more active in the box than those tested on P28, moving faster and traveling more than twice the distance (Table 3).
Table 3.
Mean group locomotor activity during habituation and quadrant crosses (QC) during social interaction ± S.E.M.
| Prenatal exposure | Sex | P28 Distance | P28 SPEED | P28QC | P42 distance❖ | P42 speed❖ | P42 QC |
|---|---|---|---|---|---|---|---|
| CH | |||||||
| NWC | M | 15.78 ± 2.68 | 0.026 ± 0.012 | 28.25 ± 5.90 | 16.97 ± 2.51 | 0.0392 ± 0.012 | 31.88 ± 5.90 |
| WC | M | 16.64 ± 2.14 | 0.027 ± 0.010 | 31.91 ± 5.04 | 19.04 ± 2.14 | 0.0465 ± 0.010 | 28.30 ± 5.28 |
| PF | |||||||
| NWC | M | 14.38 ± 1.90 | 0.024 ± 0.009 | 30.10 ± 5.28 | 21.67 ± 2.37 | 0.0391 ± 0.011 | 23.50 ± 5.91 |
| WC | M | 14.62 ± 1.90 | 0.024 ± 0.009 | 29.18 ± 5.04 | 17.01 ± 2.24 | 0.0472 ± 0.011 | 28.56 ± 5.57 |
| ET | |||||||
| NWC | M | 13.83 ± 2.37 | 0.023 ± 0.011 | 29.30 ± 5.29 | 17.52 ± 2.90 | 0.0299 ± 0.014 | 33.86 ± 6.31 |
| WC | M | 14.69 ± 2.05 | 0.024 ± 0.010 | 37.73 ± 5.03 | 20.52 ± 2.51 | 0.0464 ± 0.012 | 34.38 ± 5.90 |
| CH | |||||||
| NWC | F | 26.62 ± 2.60 | 0.025 ± 0.011 | 27.56 ± 4.95 | 28.11 ± 2.46 | 0.023 ± 0.008 | 29.33 ± 4.95 |
| WC | F | 18.85 ± 1.97^ | 0.023 ± 0.008 | 30.97 ± 4.28 | 21.80 ± 2.33^ | 0.040 ± 0.010 | 27.00 ± 4.69 |
| PF | |||||||
| NWC | F | 20.50 ± 2.61 | 0.021 ± 0.011 | 29.89 ± 4.95 | 21.45 ± 2.46 | 0.048 ± 0.011 | 33.22 ± 4.96 |
| WC | F | 21.70 ± 2.05 | 0.024 ± 0.008 | 32.82 ± 4.47 | 25.20 ± 2.34 | 0.047 ± 0.010 | 32.80 ± 4.69 |
| ET | |||||||
| NWC | F | 25.90 ± 2.33 | 0.028 ± 0.009 | 31.90 ± 4.69 | 22.13 ± 2.60 | 0.059 ± 0.011 | 25.50 ± 5.25 |
| WC | F | 21.23 ± 2.05 | 0.026 ± 0.009 | 31.85 ± 4.12 | 24.22 ± 2.45 | 0.042 ± 0.011 | 35.67 ± 4.95 |
In both sexes, habituation locomotor activity (both speed and distance) was increased in the older rats when compared to the activity of the younger offspring (P28) as denoted by
p value of <0.05. CH/WC females traveled less distance than CH/NWC females, this effect was not present in either the PF- or ET-fed females
p < 0.05.
N ranged 8-12 per sex per treatment group.
3.4.2 Locomotor activity during habituation (females)
In females, there was a significant prenatal exposure × postnatal manipulation interaction (F 2,104 = 4.118, p=0.02) on distance traveled (Table 3). Collapsed across age, the female CH/WC group was less active than the CH/NWC group (p=0.031). Whisker clipping had no effect on the PF or ET groups.
Speed was significantly affected by age (F 1,104 = 16.712, p<0.001; Table 3). Older rats traveled about twice the speed as the younger animals. Neither prenatal exposure nor postnatal manipulation had any effect on this measure.
3.4.3 Locomotor activity during social interaction (males and females)
The total number of crosses made by the experimental animal during play showed no effect of age, sex, prenatal exposure, or postnatal manipulation on this outcome in either male or female offspring (Table 3).
3.5 Gap Crossing
3.5.1 Gap crossing (males)
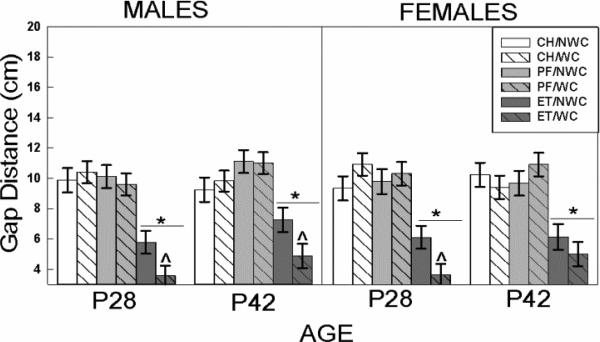
The 4-way ANOVA did not reveal either a main effect or interaction of other between subjects factors with sex, but to remain consistent with previous analyses the data set was split by sex for further assessment. In males, there was no effect of age on gap crossing outcomes. There was a significant prenatal exposure × postnatal interaction (F 2,99 = 3.66, p=0.03) when data were collapsed across ages. Both CH and PF males successfully crossed gaps averaging 8-10 cm (Figure 3). In contrast, ET/NWC pups were significantly (p<0.001 vs. CH and PF) impaired, crossing gap distances that were on average 5-6 cm. The ET/WC group was the most impaired (p=0.038 vs ET/NWC); their mean gap distance (~4 cm) was shorter than all other groups. Whisker clipping only exacerbated gap crossing deficits in prenatal ethanol exposed rats, this effect was not seen in CH or PF animals. Falls were not statistically assessed as a dependent measure due to their rarity; the majority of males failed by timing out (i.e., not crossing within the allowed 120 s). That said, a total of seven males fell off the platform during this study. The fall distribution was fairly evenly distributed with only the ET/NWC male not reporting a fall. The distribution for falls was: 1 ET/WC, 1 PF/WC, 2 PF/NWC (with 1 subject falling twice), 1 CH/WC, and 2 CH/NWC.
Figure 3. Gap Crossing.
Effects of ET and WC on gap crossing distances for males (left) and females (right) tested during pre-adolescence (P28) or mid-adolescence (P42). ET-exposed males crossed smaller gaps than CH and PF controls at both ages ( p<0.05); neonatal WC further impaired performance in ET but not PF or CH males (^ p<0.05). ET-exposed females also crossed shorter distances than CH and PF females (
p<0.05); neonatal WC further impaired performance in ET but not PF or CH males (^ p<0.05). ET-exposed females also crossed shorter distances than CH and PF females ( p<0.05). 28-day-old ET/WC females were more impaired compared to ET/NWC females (^ p<0.05). Bars depict group mean and T bars the standard error of the mean. N ranged from 8-12 per group.
p<0.05). 28-day-old ET/WC females were more impaired compared to ET/NWC females (^ p<0.05). Bars depict group mean and T bars the standard error of the mean. N ranged from 8-12 per group.
3.5.2 Gap crossing (females)
Females showed a significant prenatal × postnatal interaction × age interaction (F 2,104 =3.30 p=0.04). Post hoc breakdown of this interaction showed that groups prenatally exposed to ethanol crossed gaps significantly (p<0.001) shorter than either the CH or PF groups (ET 4-6 cm, CH and PF 9-10 cm; Figure 3). Whisker clipping further exacerbated the deficit in 28- but not 42-day-old ET-exposed females (p=0.031). There was no detectable effect of whisker clipping on the CH or PF groups at either age. Falling was a rare event for females. Only six females were reported as having fallen from the platform, with a fairly even distribution across groups: 1 ET/WC, 2 ET/NWC, 1 PF/NWC, 1 CH/WC, and 1 CH/NWC, with the PF/WC females having no falls recorded.
3.6 Elevated plus maze
3.6.1 Elevated plus maze (males)
The 4-way ANOVA did not reveal either a main effect or interaction of other between subjects factors with sex, to remain consistent with previous analyses the data set was split by sex for further assessment. In the males, there was no effect of prenatal exposure or postnatal manipulation on the proportion of time spent in open vs. closed arms; all males showed a preference for the closed arms of the maze (Table 4). A main effect of prenatal treatment was found for the total distance traveled (F 2,51 =3.52, p=0.04), with ethanol exposed rats traveling further in the elevated plus maze than the CH rats (p=0.021). PF rats were not significantly different to CH or ET. There were no between group differences on the speed of travel.
Table 4.
Mean group elevated plus maze outcomes ± S.E.M.
| Prenatal exposure | Sex | O-arm preference ratio (O-C/T) | Distance (m) | Speed (m/s) |
|---|---|---|---|---|
| CH | ||||
| NWC | M | –0.483 ± 0.172 | 19.06 ± 2.55 | 0.032 ± 0.004 |
| WC | M | –0.452 ± 0.162 | 17.60 ± 2.41 | 0.029 ± 0.004 |
| PF | ||||
| NWC | M | –0.449 ± 0.140 | 20.93 ± 2.08 | 0.034 ± 0.004 |
| WC | M | –0.445 ± 0.154 | 17.18 ± 2.28 | 0.029 ± 0.004 |
| ET | ||||
| NWC | M | –0.586 ± 0.162 | 24.07 ± 2.41* | 0.034 ± 0.004 |
| WC | M | –0.492 ± 0.162 | 24.28 ± 2.41* | 0.031 ± 0.004 |
| CH | ||||
| NWC | F | –0.492 ± 0.162 | 24.28 ± 2.41 | 0.031 ± 0.004 |
| WC | F | –0.351 ± 0.179 | 20.00 ± 2.92^ | 0.033 ± 0.004^ |
| PF | ||||
| NWC | F | –0.385 ± 0.189 | 26.99 ± 3.08 | 0.044 ± 0.005 |
| WC | F | –0.200 ± 0.179 | 24.74 ± 2.92^ | 0.024 ± 0.004^ |
| ET | ||||
| NWC | F | –0.290 ± 0.179 | 22.64 ± 2.92 | 0.038 ± 0.004 |
| WC | F | –0.385 ± 0.179 | 21.21 ± 2.92^ | 0.035 ± 0.004^ |
ET males traveled further distances than CH males
p < 0.05.
WC females traveled shorter distances and slower than NWC females while in the elevated plus maze
p < 0.05.
N ranged from 8 to 11 per sex per treatment group.
3.6.2 Elevated plus maze (females)
No effect of prenatal exposure was found regarding arm preference; females from all groups spent more time in the closed arms than in the open (Table 4). There was a main effect of postnatal whisker clipping on both distance traveled (F 1,50 = 4.35, p=0.04), and speed (F 1,50 = 4.22, p=0.05). Whisker clipping induced a modest hypo-activity in females regardless of age or prenatal exposure; WC rats traveled shorter distances (p=0.042) and moved more slowly (p=0.045) than their NWC counterparts.
4. DISCUSSION
Both prenatal exposure to ethanol and postnatal whisker clipping affected social behavior and gap crossing. These effects were apparent despite a lack of effect of either manipulation on whisker length or anxiety measures. Outcomes were age- and/or sex-dependent.
4.1 Ethanol effect
Rats exposed to ethanol during the prenatal period showed an overall decrease in play fighting and distance crossed on GC task (somatosensory performance) regardless of sex or age at testing. In contrast, chasing and social motivation showed age- and sex-dependent outcomes. Prenatal exposure to ethanol has a bidirectional effect on chasing with an increase in this behavior in 28-day-old females and a decrease in 42-day-old males. Motivation for social interaction, on the other hand, was only decreased in 42-day-old ET females.
Other reports of social interaction following prenatal and early postnatal ethanol exposure are varied. At least some of this variability in outcome is likely due to differences in exposure methodology (i.e. dose, timing, and duration of the ethanol exposure as well as differences in social interaction testing and dependent measures [2, 4, 9, 10, 59]. For instance, adolescent animals prenatally exposed to an acute dose of ethanol on G7 showed decreased social interactions (playing fighting and social motivation) [9], changing the timing of exposure to G12 decreased playing fighting in males only and decreased social motivation in males and females [9]. Methodological differences in the dose and duration of ethanol exposure also alters social outcomes during adolescence and into adulthood. Interestingly, increased frequency in measures of play interactions were reported both by Royalty [10] and Hamilton et al. [58] after prenatal exposure to a low dose and by Kelly et al. [2, 58] after exposure to a higher dose during the prenatal and early neonatal period. Thus, timing, dose, and duration of the ethanol exposure define the way in which ethanol disrupts social interaction during adolescence.
Meyer and Riley as well as Kelly and Dillingham have both reported ethanol-induced sexually dimorphic effects in play interaction [4, 59]. A similar effect was seen in the current study within the chasing measure; this was increased in 28-day-old females but decreased in 42-day-old males. Similar sexually dimorphic effects were also present in the social motivation measure such that older females but not males displayed ethanol-induced social avoidance in the older cohorts. The reason(s) for these sexually dimorphic changes is unclear, but it is possible that these behavioral patterns may be related to females reaching hormonal and physical markers of puberty prior to males [60]. Prenatal ethanol exposure may shift the onset of these developmental stages and thereby alter sex-hormones and brain development [61, 62].
As predicted by our hypothesis, prenatal ethanol exposure also impaired performance on the gap crossing task. The initial gaps are short and therefore can likely be seen by the animals, and/or felt with the paw or nose. With increasing gap distance, the animal is forced to use the mystacial vibrissae (long whiskers on the snout) to feel where the escape platform is. There was no decrease in whisker length in ET animals suggesting that failure to cross the gap was not because their whiskers were not long enough to feel the gap. ET animals also showed no evidence of anxiety in the elevated plus maze suggesting that they do not fail to cross the gap due to anxiety. Taken together with other reports of prenatal ethanol-induced impairments of sensory detection and processing [2, 63], our data shows a functional outcome of the prenatal ethanol exposure-induced disruption of somatosensory structure and function.
4.2 Whisker clip effect
Unilateral whisker clipping alone had relatively little effect on social behavior; it reduced sniffing in males and in 28-day-old females but did not alter any other social behaviors. Whisker clipping also had an effect on locomotor activity in females; WC offspring of CH dams were mildly hypoactive during habituation to the social interaction test box, and all WC females were hyperactive in the elevated plus maze relative to NWC animals. The reasons for these mixed changes in activity in the female offspring are unclear.
Intriguingly, whisker-clipping had no effect on gap crossing outcomes in control animals. These behavioral findings are contrary to those reported by Lee et al. [38] who report increased social interactions (including sniffing) and activity as well as impaired gap crossing performance. Those animals were whisker-clipped bilaterally each day during the first 3 days of life, resulting in bilateral reduction in sensory input during this time. The intermittent and unilateral nature of the clipping in the current study would allow for more sensory stimulation, albeit abnormal, which may contribute to the differences in outcome between the two studies.
4.3 Ethanol × whisker clip interaction effect
In animals that were prenatally exposed to ethanol, whisker clipping further exacerbated ethanol-induced deficits in both play fighting and in the gap crossing task. This was seen at both ages in males, but in the females only the younger cohort showed an exacerbation of ethanol-induced deficits by whisker clipping. Thus, prenatal ethanol exposure appears to increase vulnerability to mild sensory impoverishment, which could be indicative of potentiated disorganization/dysfunction within the somatosensory system.
This apparent increase in vulnerability to mild sensory impoverishment in ethanol-exposed animals is somewhat similar to the increased vulnerability to chronic mild stress seen after prenatal ethanol exposure [64-67] and to mild hypoxic events following exposure to ethanol during the first neonatal week of life [68]. In all of these cases mild manipulations that do not cause significant effects in control animals alter outcomes in ET animals to a magnitude not anticipated by simple cumulative effects suggesting that programming during early development is a major factor in late life outcomes.
In our study, males appear to have a persistent sensitivity to the combined the insult of prenatal ethanol and mild early sensory impoverishment as evidenced in the social interaction and gap crossing performance of the older cohort. This may be due to sex dependent factors that make the male brain either preferentially vulnerable to ethanol-induced damage or lacking some factor that allows female offspring to recover. This is in line with published reports showing prenatal ethanol can have more of a negative effect on males compared to females in several brain and behavioral outcomes. A reduction in long term potentiation in the dentate gyrus of the hippocampus, reduced c-fos activation in the nucleus accumbens [2, 67, 68], and increased corticotropin-releasing factor in the paraventricular nucleus of the hypothalamus [69] were present in adolescent males following prenatal ethanol, but not females. Such effects of prenatal alcohol on the brain may contribute to some of the ethanol-induced sex-dependant behavioral deficits [53-56]. Further studies examining fetal ethanol's effects in other brain regions should make a point to avoid the experimental bias of disregarding sex as a viable and important independent variable in order to better understand the differences between the two sexes.
4.4 Age effect
In general, social play measures tend to increase in frequency from P18 to peak around P30-40, waning thereafter as they approach adulthood (~P50-60) [69]. The female social play data from the current study appears to follow this pattern with the younger P28 females engaged in more social interactive activities than older females. However, the male social play results show a consistent plateau, suggesting that they may have reached their peak at P28 and have not yet begun their decline at P42. This would follow the pattern of males taking longer to reach maturity landmarks in relation to female offspring [60], however, recent work by Hamilton et al. [70] shows persistence of fetal ethanol-induced deficits well into adulthood in male offspring. Thus, although females do tend to mature faster than males, maturation may not be enough to explain the persistence of the behavioral deficits in the male offspring.
5. Conclusion
In conclusion, chronic prenatal ethanol exposure has a negative effect on social interaction and somatosensory performance during adolescence, and sensory impoverishment worsens these outcomes in ethanol-exposed animals while only having subtle effects in control animals. It should also be noted that although the sensory impoverishment applied in this experiment is occurring during a period of brain development which is roughly congruent with the human 3rd trimester development [71], this does not unequivocally negate the potential application of these findings regarding human neonate development. Firstly, brain development in premature infants would fall into the 3rd trimester developmental period. Often these children are isolated within isolettes with limited tactile or abnormal tactile stimulation. Secondly, there are some reports that prenatal exposure to ethanol may be correlated with prematurity [72, 73], although other studies refute this [74]. Finally, there is evidence that somatosensory functional connectivity (i.e. dendritic remodeling and maintenance) is activity/stimuli dependent and particularly active during neonatal development with refinement ongoing into adolescence [75, 76], suggesting that this developmental window may be more extensive in humans.
Taken together, these findings stress the importance of somatosensory function on normal social development. These findings also suggest that therapies that manipulate the structure and/or function of the somatosensory system may improve social outcomes and somatosensory performance in fetal alcohol populations.
Highlights.
-
1)
Prenatal ethanol exposure impairs social interaction and somatosensory performance.
-
2)
Neonatal sensory impoverishment potentiates prenatal ethanol-induced behavior deficits.
-
3)
Interactions of ethanol- and impoverishment-induced deficits are age and sex-dependent.
-
4)
Somatosensory function plays an important role in normal social development.
-
5)
Enhancement of somatosensory development may improve fetal alcohol outcomes.
Acknowledgments
The authors thank Celina Tran, Kay Kulason, and Dr. Finney George, M.D. for technical assistance. This research was supported by the National Institute of Alcohol Abuse and Alcoholism (AA018693to SM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res. 2010;34:1022–32. doi: 10.1111/j.1530-0277.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- 2.Charles Lawrence R, Cale Bonner H, Newsom RJ, Kelly SJ. Effects of alcohol exposure during development on play behavior and c-fos expression in response to play behavior. Behav Brain Res. 2008;188:209–18. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- 5.Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: Effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- 6.Rema V, Ebner FF. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. J Neurosci. 1999;19:10993–1006. doi: 10.1523/JNEUROSCI.19-24-10993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265:1961–7. [PubMed] [Google Scholar]
- 8.Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Neurobehavioral effects of prenatal alcohol: Part III. PLS analyses of neuropsychologic tests. Neurotoxicol Teratol. 1989;11:493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 9.Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: Effects of age, sex, and timing of exposure. Behav Brain Res. 2011;216:358–64. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royalty J. Effects of prenatal ethanol exposure on juvenile play-fighting and postpubertal aggression in rats. Psychol Rep. 1990;66:551–60. doi: 10.2466/pr0.1990.66.2.551. [DOI] [PubMed] [Google Scholar]
- 11.Roebuck TM, Mattson SN, Riley EP. Interhemispheric transfer in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:1863–71. doi: 10.1097/01.ALC.0000042219.73648.46. [DOI] [PubMed] [Google Scholar]
- 12.Varlinskaya EI, Mooney SM. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav Brain Res. 2014;261:106–9. doi: 10.1016/j.bbr.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whaley SE, O'Connor M, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res. 2001;25:1018–24. [PubMed] [Google Scholar]
- 14.Goodlett CR, Gilliam DM, Nichols JM, West JR. Genetic influences on brain growth restriction induced by development exposure to alcohol. Neurotoxicology. 1989;10:321–34. [PubMed] [Google Scholar]
- 15.Jacobson JL, Jacobson SW, Sokol RJ, Ager JJ. Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res. 1998;22:345–51. doi: 10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson JL, Jacobson SW, Sokol RJ. Increased vulnerability to alcohol-related birth defects in the offspring of mothers over 30. Alcohol Clin Exp Res. 1996;20:359–63. doi: 10.1111/j.1530-0277.1996.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 17.Streissguth AP, Dehaene P. Fetal alcohol syndrome in twins of alcoholic mothers: Concordance of diagnosis and IQ. Am J Med Genet. 1993;47:857–61. doi: 10.1002/ajmg.1320470612. [DOI] [PubMed] [Google Scholar]
- 18.Warren KR, Foudin LL. Alcohol-related birth defects--the past, present, and future. Alcohol Res Health. 2001;25:153–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Maier SE, West JR. Alcohol and nutritional control treatments during neurogenesis in rat brain reduce total neuron number in locus coeruleus, but not in cerebellum or inferior olive. Alcohol. 2003;30:67–74. doi: 10.1016/s0741-8329(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor M, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15:225–34. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- 21.Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol- exposed children. Alcohol Clin Exp Res. 1999;23:1070–6. [PubMed] [Google Scholar]
- 22.Blanchard BA, Hannigan JH. Prenatal ethanol exposure: Effects on androgen and nonandrogen dependent behaviors and on gonadal development in male rats. Neurotoxicol Teratol. 1994;16:31–9. doi: 10.1016/0892-0362(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 23.Elis J, Krsiak M. Proceedings: Effect of alcohol administration during pregnancy on social behaviour of offsprings in mice. Act Nerv Super (Praha) 1975;17:281–2. [PubMed] [Google Scholar]
- 24.Boggan WO, Randall CL, Dodds HM. Delayed sexual maturation in female C57BL/6J mice prenatally exposed to alcohol. Res Commun Chem Pathol Pharmacol. 1979;23:117–25. [PubMed] [Google Scholar]
- 25.McGivern RF, Handa RJ. Prenatal exposure to drugs of abuse: Methodological considerations and effects on sexual differentiation. NIDA Res Monogr. 1996;164:78–124. [PubMed] [Google Scholar]
- 26.Margret CP, Li CX, Elberger AJ, Matta SG, Chappell TD, Waters RS. Prenatal alcohol exposure alters the size, but not the pattern, of the whisker representation in neonatal rat barrel cortex. Exp Brain Res. 2005;165:167–78. doi: 10.1007/s00221-005-2287-9. [DOI] [PubMed] [Google Scholar]
- 27.Margret CP, Li CX, Chappell TD, Elberger AJ, Matta SG, Waters RS. Prenatal alcohol exposure delays the development of the cortical barrel field in neonatal rats. Exp Brain Res. 2006;172:1–13. doi: 10.1007/s00221-005-0319-0. [DOI] [PubMed] [Google Scholar]
- 28.Miller MW. Relationship of the time of origin and death of neurons in rat somatosensory cortex: Barrel versus septal cortex and projection versus local circuit neurons. J Comp Neurol. 1995;355:6–14. doi: 10.1002/cne.903550104. [DOI] [PubMed] [Google Scholar]
- 29.Oladehin A, Margret CP, Maier SE, Li CX, Jan TA, Chappell TD, Waters RS. Early postnatal alcohol exposure reduced the size of vibrissal barrel field in rat somatosensory cortex (SI) but did not disrupt barrel field organization. Alcohol. 2007;41:253–61. doi: 10.1016/j.alcohol.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powrozek TA, Zhou FC. Effects of prenatal alcohol exposure on the development of the vibrissal somatosensory cortical barrel network. Brain Res Dev Brain Res. 2005;155:135–46. doi: 10.1016/j.devbrainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Panksepp J, Normansell L, Cox JF, Siviy SM. Effects of neonatal decortication on the social play of juvenile rats. Physiol Behav. 1994;56:429–43. doi: 10.1016/0031-9384(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 32.Pellis SM, McKenna MM, Field EF, Pellis VC, Prusky GT, Whishaw IQ. Uses of vision by rats in play fighting and other close-quarter social interactions. Physiol Behav. 1996;59:905–13. doi: 10.1016/0031-9384(95)02162-0. [DOI] [PubMed] [Google Scholar]
- 33.Siviy SM, Panksepp J. Sensory modulation of juvenile play in rats. Dev Psychobiol. 1987;20:39–55. doi: 10.1002/dev.420200108. [DOI] [PubMed] [Google Scholar]
- 34.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree J. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology (Berl) 1995;117:225–31. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- 35.Deak T, Panksepp J. Play behavior in rats pretreated with scopolamine: Increased play solicitation by the non-injected partner. Physiol Behav. 2006;87:120–5. doi: 10.1016/j.physbeh.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Blue ME, Erzurumlu RS, Jhaveri S. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–9. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- 37.Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: A study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22:8541–52. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee LJ, Chen WJ, Chuang YW, Wang YC. Neonatal whisker trimming causes long-lasting changes in structure and function of the somatosensory system. Exp Neurol. 2009;219:524–32. doi: 10.1016/j.expneurol.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Chu YF, Yen CT, Lee LJ. Neonatal whisker clipping alters behavior, neuronal structure and neural activity in adult rats. Behav Brain Res. 2013;238:124–33. doi: 10.1016/j.bbr.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 41.Van d. L., Woolsey TA. Somatosensory cortex: Structural alterations following early injury to sense organs. Science. 1973;179:395–8. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- 42.Granato A, Santarelli M, Sbriccoli A, Minciacchi D. Multifaceted alterations of the thalamo cortico-thalamic loop in adult rats prenatally exposed to ethanol. Anat Embryol (Berl) 1995;191:11–23. doi: 10.1007/BF00215293. [DOI] [PubMed] [Google Scholar]
- 43.Vingan RD, Dow-Edwards D, Riley EP. Cerebral metabolic alterations in rats following prenatal alcohol exposure: A deoxyglucose study. Alcohol Clin Exp Res. 1986;10:22–6. doi: 10.1111/j.1530-0277.1986.tb05607.x. [DOI] [PubMed] [Google Scholar]
- 44.Miller MW, Dow-Edwards D. Structural and metabolic alterations in rat cerebral cortex induced by prenatal exposure to ethanol. Brain Res. 1988;474:316–26. doi: 10.1016/0006-8993(88)90445-3. [DOI] [PubMed] [Google Scholar]
- 45.Miller MW, Dow-Edwards D. Vibrissal stimulation affects glucose utilization in the trigeminal/somatosensory system of normal rats and rats prenatally exposed to ethanol. J Comp Neurol. 1993;335:283–4. doi: 10.1002/cne.903350211. [DOI] [PubMed] [Google Scholar]
- 46.Rema V, Armstrong-James M, Ebner FF. Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J Neurosci. 1998;18:10196–206. doi: 10.1523/JNEUROSCI.18-23-10196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller MW. Circadian rhythm of cell proliferation in the telencephalic ventricular zone: Effect of in utero exposure to ethanol. Brain Res. 1992;595:17–24. doi: 10.1016/0006-8993(92)91447-m. [DOI] [PubMed] [Google Scholar]
- 48.Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: The effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav Neurosci. 2007;121:1293–305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbey H, Howard E. Statistical procedure in developmental studies on species with multiple offspring. Dev Psychobiol. 1973;6:329–35. doi: 10.1002/dev.420060406. [DOI] [PubMed] [Google Scholar]
- 50.Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci. 2012;34:115–28. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mooney SM, Miller MW. Prenatal exposure to ethanol affects postnatal neurogenesis in thalamus. Exp Neurol. 2010;223:566–73. doi: 10.1016/j.expneurol.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: Role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–82. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 53.Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–20. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 54.Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2012;62:1607–18. doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–75. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- 56.Zimmerberg B, Sukel HL, Stekler JD. Spatial learning of adult rats with fetal alcohol exposure: Deficits are sex-dependent. Behav Brain Res. 1991;42:49–56. doi: 10.1016/s0166-4328(05)80039-7. [DOI] [PubMed] [Google Scholar]
- 57.Blanchard BA, Riley EP, Hannigan JH. Deficits on a spatial navigation task following prenatal exposure to ethanol. Neurotoxicol Teratol. 1987;9:253–8. doi: 10.1016/0892-0362(87)90010-9. [DOI] [PubMed] [Google Scholar]
- 58.Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook F, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol Teratol. 1994;16:377–84. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 60.Vetter-O'Hagen C, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012;54:523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–40. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- 62.McGivern RF, McGeary J, Robeck S, Cohen S, Handa RJ. Loss of reproductive competence at an earlier age in female rats exposed prenatally to ethanol. Alcohol Clin Exp Res. 1995;19:427–33. doi: 10.1111/j.1530-0277.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 63.Rema V, Armstrong-James M, Ebner FF. Experience-dependent plasticity is impaired in adult rat barrel cortex after whiskers are unused in early postnatal life. J Neurosci. 2003;23:358–66. doi: 10.1523/JNEUROSCI.23-01-00358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010;34:633–45. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: Foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carter M, Fields L, Hawkey A, Barron S. The combination of ETOH and hypoxia produces behavioral deficits in a 3rd trimester rodent model. Alcoholism: Clinical and Experimental Research. 2013;37:126a. [Google Scholar]
- 69.Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–32. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 70.Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, Savage DD. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Passingham RE. Rates of brain development in mammals including man. Brain Behav Evol. 1985;26:167–75. doi: 10.1159/000118773. [DOI] [PubMed] [Google Scholar]
- 72.Albertsen K, Andersen AM, Olsen J, Gronbaek M. Alcohol consumption during pregnancy and the risk of preterm delivery. Am J Epidemiol. 2004;159:155–161. doi: 10.1093/aje/kwh034. [DOI] [PubMed] [Google Scholar]
- 73.O'Leary CM, Nassar N, Kurinczuk JJ, Bower C. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG. 2009;116:390–400. doi: 10.1111/j.1471-0528.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 74.Cornman-Homonoff J, Kuehn D, Aros S, Carter TC, Conley MR, Troendle J, Cassorla F, Mills JL. Heavy prenatal alcohol exposure and risk of stillbirth and preterm delivery. J Matern Fetal Neonatal Med. 2012;25:860–863. doi: 10.3109/14767058.2011.587559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]