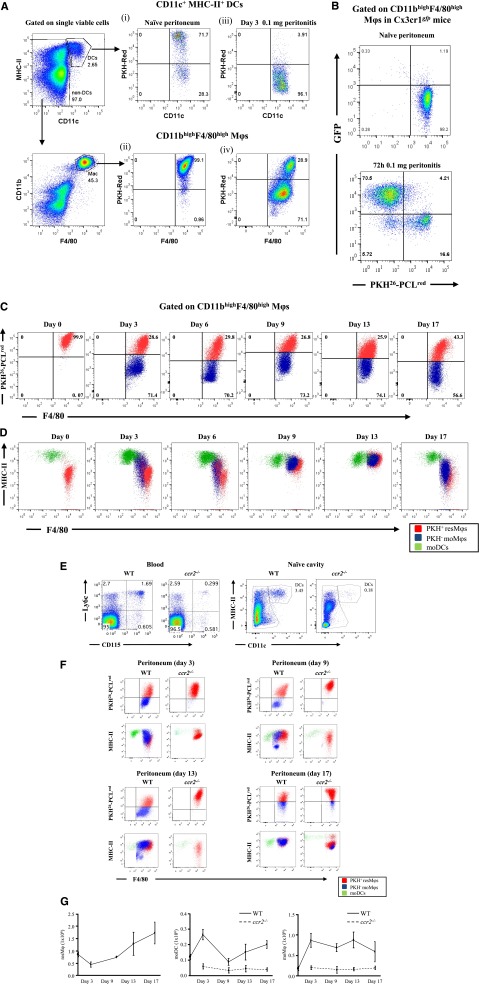
Figure 2.
Temporal profiles of mononuclear phagocytes and DCs throughout inflammation, resolution, and postresolution/adaptive immunity phase. The gating strategy in supplemental Figure 4 was used to identify Mφ and DC populations in the peritoneal cavity of naïve mice. Using this approach, (A) the cell tracker dye PKH26-PCLred was injected into the cavity of mice and its labeling of tissue-resident DCs and resMφ determined in the naïve peritoneum (A, panels i-ii, respectively). These mice were then injected with 0.1 mg of zymosan, revealing (A, panel iii) the disappearance of DCs from the naïve peritoneum after inflammation and the presence of both (A, panel iv) PKH26-PCLred-positive resMφs and PKH26-PCLred-negative moMφs 72 hours post-zymosan. The origin of the latter as being Ly6chi-derived was confirmed using (B) CX3CRgfp mice. (C) The temporal and relative changes of resMφs vs moMφs (Ly6chi–monocyte-derived) from onset (4 hours), classic resolution (48-72 hours) and postresolution from day 6 onwards are shown. These data were further back-gated onto (D) MHC-II vs F4/80 to depict the overall temporal changes of mononuclear phagocytes and DCs throughout and after resolving inflammation. Further experiments were carried out using (E-F) ccr2−/− mice to prove the ly6chi origin of moMφs and MoDCs throughout resolution and postresolution with the (G) temporal profiles of resMφs, moMφs, and MoDCs shown. Data are presented as mean ± SEM for n = 6 mice/group.