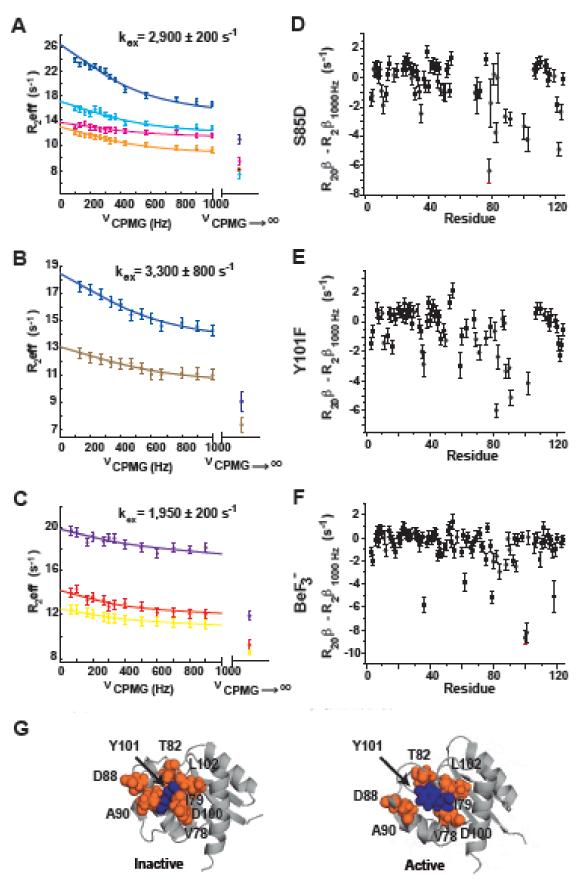
Figure 1. Y101 dynamics is kinetically independent from the inactive to active transition.
(A-C) 15N-CPMG relaxation dispersion profiles and R20β values for residues undergoing exchange between the inactive and active states (kex) are compared to the true exchange-free transverse relaxation rates determined independently (R20β)31 for A) S85D, B) Y101F, and C) BeF3− -activated NtrCR. The CPMG curves refer to residues 78 (cyan), 79 (yellow), 82 (magenta), 88 (orange), 90 (light brown), 100 (purple), 101 (red), 102 (blue), and the independently determined (R20β) are shown as R2eff at νCPMG→∞. The difference between R20β and R2β1000Hz is plotted for all amides in panels (D-F), showing that many residues sense a second fast exchange process that cannot be suppressed by the applied CPMG field strength. G) The residues with exchange from both processes, the inactive/active interconversion and this second faster process (orange) are clustered around Y101 (blue) in both the inactive (pdbID 1DC7) and active (pdbID 1DC8) state structures.