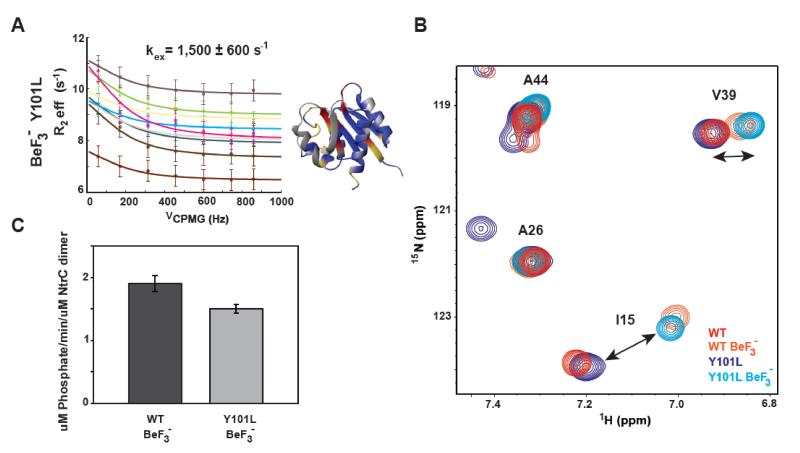
Figure 4. Mutating the conserved aromatic does not prohibit activation of the receiver domain or NtrC’s downstream ATPase activity.
A) 15N-CPMG NMR relaxation dispersion profiles for the BeF3− -activated Y101L NtrCR mutant fit to a single exchange process of inactive/active interconversion. Residues whose dispersion was fit globally to determine the rate of exchange (kex) between the inactive and active forms of the protein are shown on the structure (pdbID 1DC8) in red. Yellow denotes residues whose peaks are severely exchanged broadened, residues that are flat/non-exchanging are colored in blue, and unassigned/proline/overlapped residues are in gray. Dispersion data is color coded as follows: 6 (ivory), 10 (burnt sienna), 12 (slate), 53 (tan), 64 (brown), 78 (cyan), 81 (green), 82 (magenta), 96 (purple). B) 15N-HSQC overlay of WT, Y101L, BeF3− -activated WT, and BeF3− -activated Y101L NtrCR highlighting that the Y101L mutant has the characteristic peak shifts associated with the active state upon the addition of BeF3−. C) Full length NtrC ATPase activity assays comparing the net activity of BeF3− -activated WT (dark grey) and Y101L (light grey).