Abstract
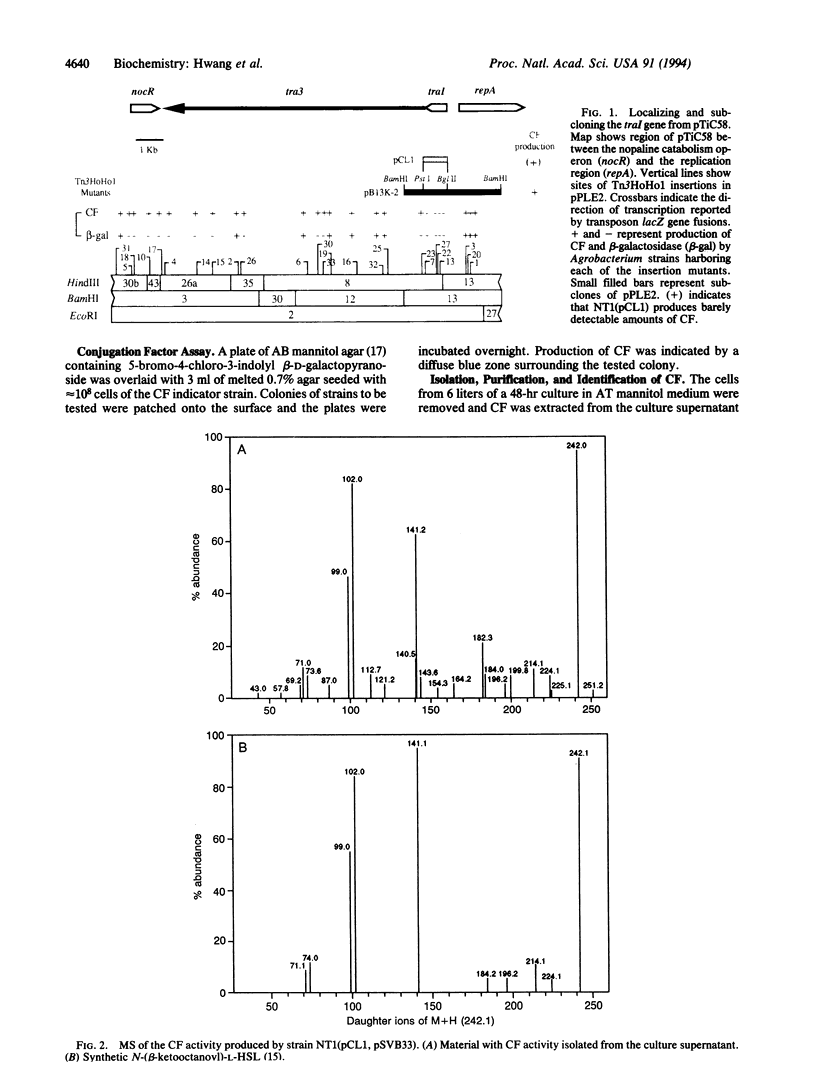
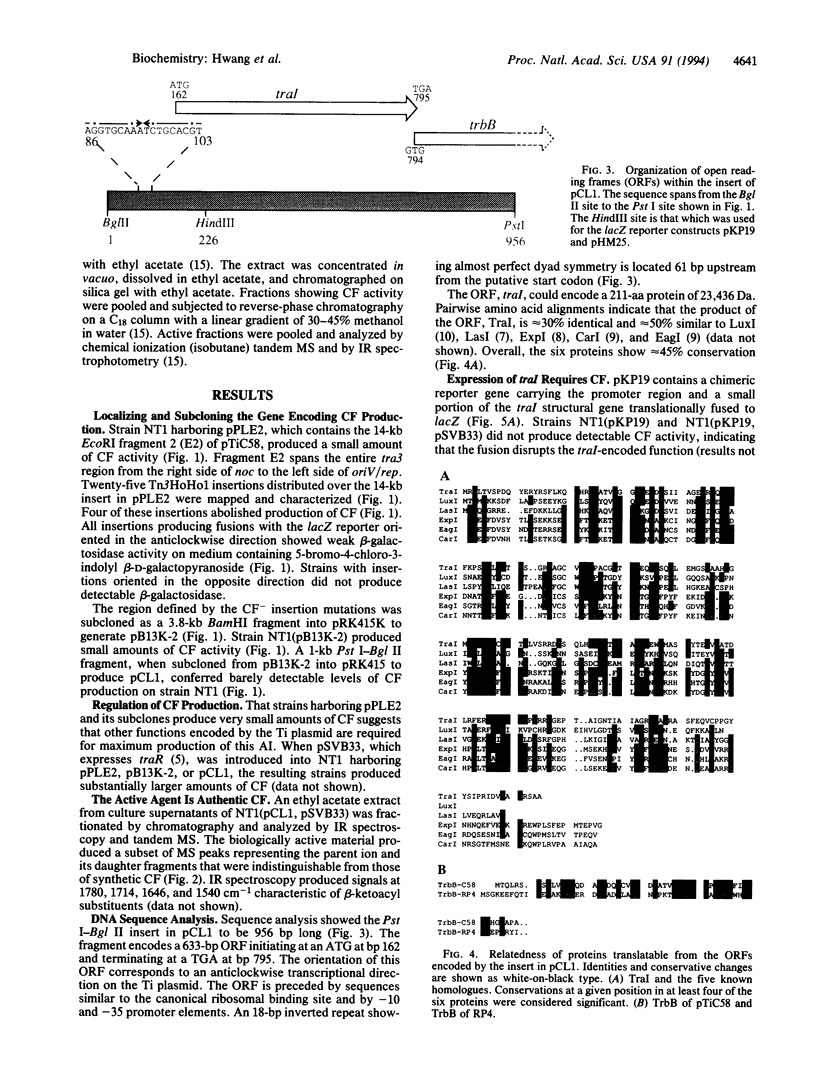
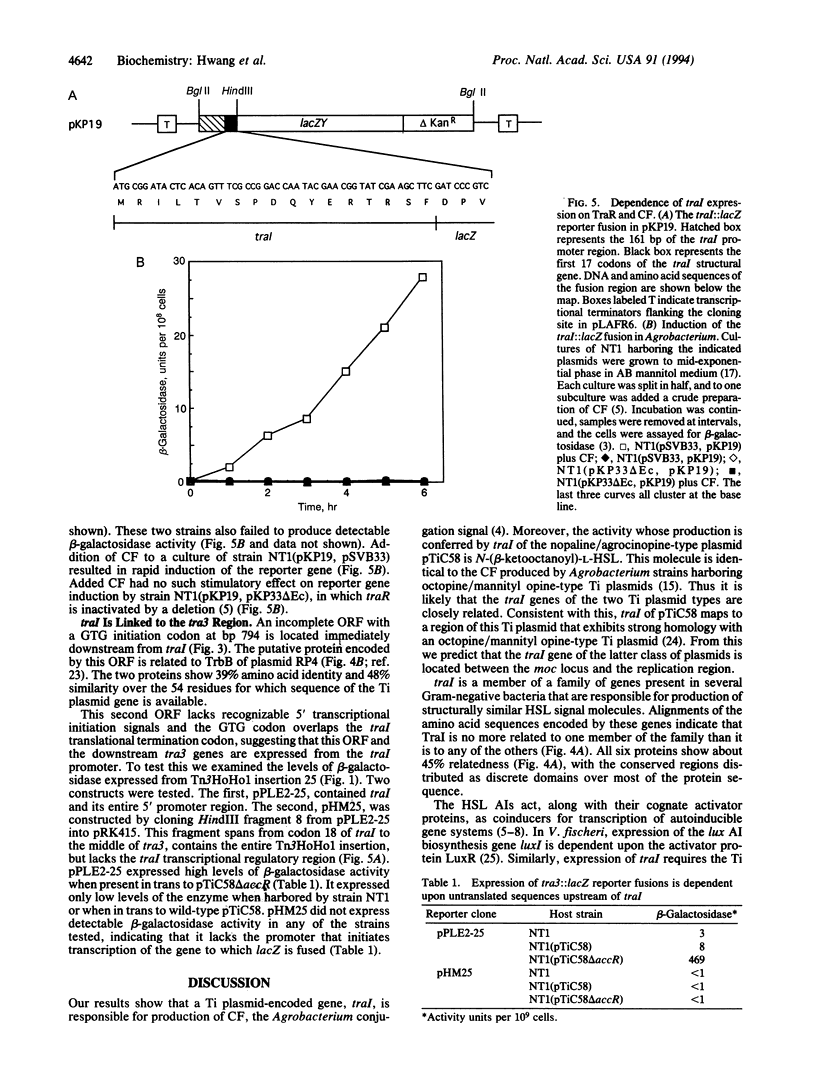
Conjugal transfer of the nopaline-type Agrobacterium Ti plasmid pTiC58 is regulated by a transcriptional activator, TraR, and a diffusible signal molecule, conjugation factor (CF). CF is a member of a family of substituted homoserine lactones (HSLs) that act as coinducers for regulating gene expression in diverse Gram-negative bacteria by a mechanism called autoinduction. In Vibrio fischeri HSL production is conferred by the luxI gene. Homologues of this gene are responsible for HSL production by other Gram-negative bacteria. A gene that we call traI, conferring production of material with CF activity, was localized to a 1-kb region at the upstream end of tra3 of pTiC58. Spectroscopy showed that the activity was authentic CF. Sequence analysis showed that traI could encode a protein of 211 amino acids, TraI, that is related to the proteins responsible for HSL production by other bacteria. A second, partial open reading frame immediately downstream of traI could encode a protein related to TrbB of plasmid RP4, which is required for conjugal transfer. Transcription of traI and of the downstream tra3 genes requires TraR and CF and initiates from the traI promoter. The results show that traI is responsible for CF production, that it is the first gene of the tra3 operon, and that expression of this operon is regulated by autoinduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bainton N. J., Stead P., Chhabra S. R., Bycroft B. W., Salmond G. P., Stewart G. S., Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992 Dec 15;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. O., Devine J. H., Heckel R. C., Lin J. W., Shadel G. S. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolumin Chemilumin. 1989 Jul;4(1):326–341. doi: 10.1002/bio.1170040145. [DOI] [PubMed] [Google Scholar]
- Beck von Bodman S., Hayman G. T., Farrand S. K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. M., Farrand S. K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992 Oct;174(19):6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987 Dec 23;15(24):10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler G., Depicker A., Maenhaut R., Villarroel R., Van Montagu M., Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981 Oct 25;152(2):183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Lessl M., Balzer D., Pansegrau W., Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992 Oct 5;267(28):20471–20480. [PubMed] [Google Scholar]
- Meyn M. S. High spontaneous intrachromosomal recombination rates in ataxia-telangiectasia. Science. 1993 May 28;260(5112):1327–1330. doi: 10.1126/science.8493577. [DOI] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., Greenberg E. P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper K. R., Beck von Bodman S., Farrand S. K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993 Apr 1;362(6419):448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- Pirhonen M., Flego D., Heikinheimo R., Palva E. T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993 Jun;12(6):2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme L. G., Mindrinos M. N., Panopoulos N. J. Genetic and transcriptional organization of the hrp cluster of Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1991 Jan;173(2):575–586. doi: 10.1128/jb.173.2.575-586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S., Winson M. K., Chan P. F., Bainton N. J., Birdsall M., Reeves P. J., Rees C. E., Chhabra S. R., Hill P. J., Throup J. P. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol Microbiol. 1993 Nov;10(3):511–520. doi: 10.1111/j.1365-2958.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M. lacZY gene fusion cassettes with KanR resistance. Nucleic Acids Res. 1988 Apr 25;16(8):3587–3587. doi: 10.1093/nar/16.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. H., Kerr A. A diffusible compound can enhance conjugal transfer of the Ti plasmid in Agrobacterium tumefaciens. J Bacteriol. 1991 Mar;173(6):1867–1872. doi: 10.1128/jb.173.6.1867-1872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Murphy P. J., Kerr A., Tate M. E. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993 Apr 1;362(6419):446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- von Bodman S. B., McCutchan J. E., Farrand S. K. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol. 1989 Oct;171(10):5281–5289. doi: 10.1128/jb.171.10.5281-5289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]