Figure 5.
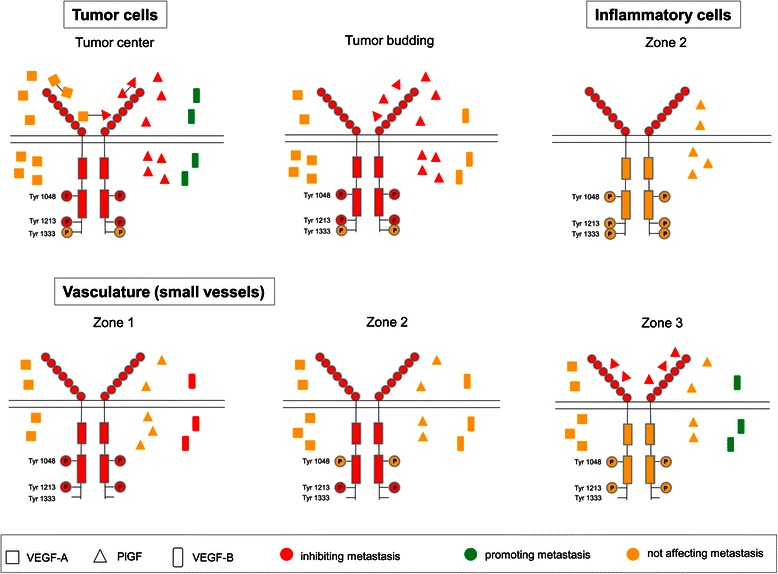
Schematic presentation of VEGFR-1 activation in CC tissue and its association with metastasis. VEGF produced in the tumor center and PlGF produced intratumorally and in tumor budding regions by tumor cells have an autocrine affinity for their receptor VEGFR-1. Subsequent PlGF-mediated receptor activation by autophosphorylation at Tyr1048 and Tyr1213 is a potential signaling pathway, which in turn seems to inhibit distant metastasis and, in regions of tumor budding, additionally lymph node metastasis. This autocrine link could be supported by possible formation of PlGF-VEGF heterodimers and PlGF-PlGF homodimers, which are known to have anti-metastatic properties. In contrast, in order to enhance their potential for distant metastasis, tumor cells in the tumor center produce paracrine-acting VEGF-B. Inflammatory cell associated VEGFR-1 expression in the invasive front (zone 2) without accompanying autophosphorylation could inhibit distant metastasis possibly by acting as a decoy and scavenger receptor. In small vessels located intratumorally (zone 1) paracrine-mediated receptor autophosphorylation at Tyr1048 and Tyr1213 and paracrine-acting VEGF-B production appear to be associated with a non-metastatic phenotype. In small vessels located along the invasive front (zone 2) paracrine-mediated receptor autophosphorylation at Tyr1213 may cause inhibition of metastasis. Autocrine-acting PlGF production by small vessels located extratumorally (zone 3) appears to be associated with a non-metastatic phenotype. In contrast, VEGF-B-expressing extratumoral small vessels correlate with distant metastasis.
