Figure 8.
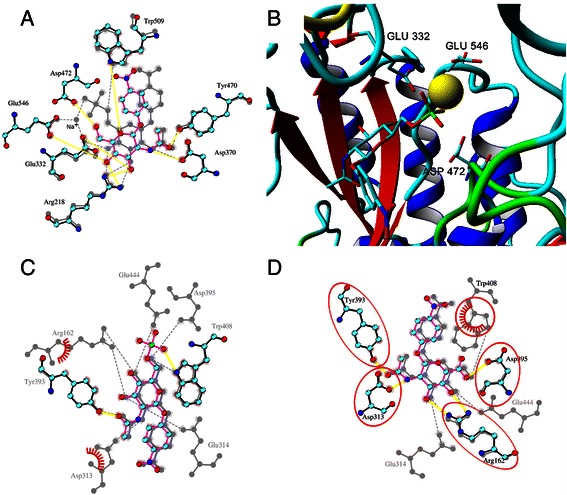
Docking of C-6 modified substrates. A. Overlay of the active sites of TfHex with docked pNP-GlcNAc (2; vivid color, yellow hydrogen bonds) and pNP-GlcNAc-sulfate (6; grey). Sodium ion in the active site with the sulfated substrate is shown. B. TfHex active site with sulfated substrate 6 in the active site. Sodium ion penetrated in the active site from water solution is shown by yellow ball. Negatively charged amino acids close to the sulfo-group are shown and labeled. Distance from Cδ atom of Glu 332 and Glu 546 to sulfur atom of substrate is 0.537-0.602 and 0.465-0.609 nm, respectively, from Cε atom of Asp 472 to sulfur atom of substrate it is 0.619-0.819 nm. C. Overlay of the active site of S. plicatus hexosaminidase with docked sulfated compound (6; vivid color) and pNP-GlcNAc (2; grey). D. Overlay of the active site of S. plicatus hexosaminidase with docked pNP-GlcNAc-uronate (5; vivid) and pNP-GlcNAc (2; grey).
