Scheme 4.
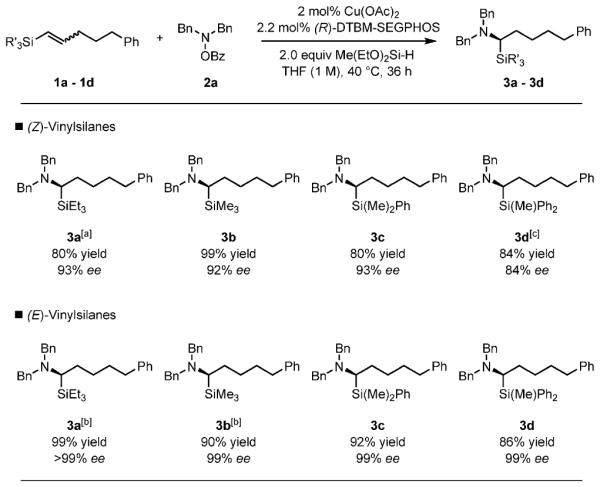
Influence of the silyl group and olefin geometry on yield and enantioselectivity. Reaction conditions: 1a–1d (1 mmol), 2a (1.2 mmol), Cu(OAc)2 (0.02 mmol), (R)-DTBM-SEGPHOS (0.022 mmol), THF (1 mL), 40 8C, 36 h. Yields are of isolated products (average of two runs). [a] Cu(OAc)2 (0.04 mmol), (R)-DTBM-SEGPHOS (0.044 mmol). [b] 8 h. [c] Cu(OAc)2 (0.04 mmol), (R)-DTBM-SEGPHOS (0.044 mmol), THF (0.5 mL, 2 m). Bn = benzyl.
