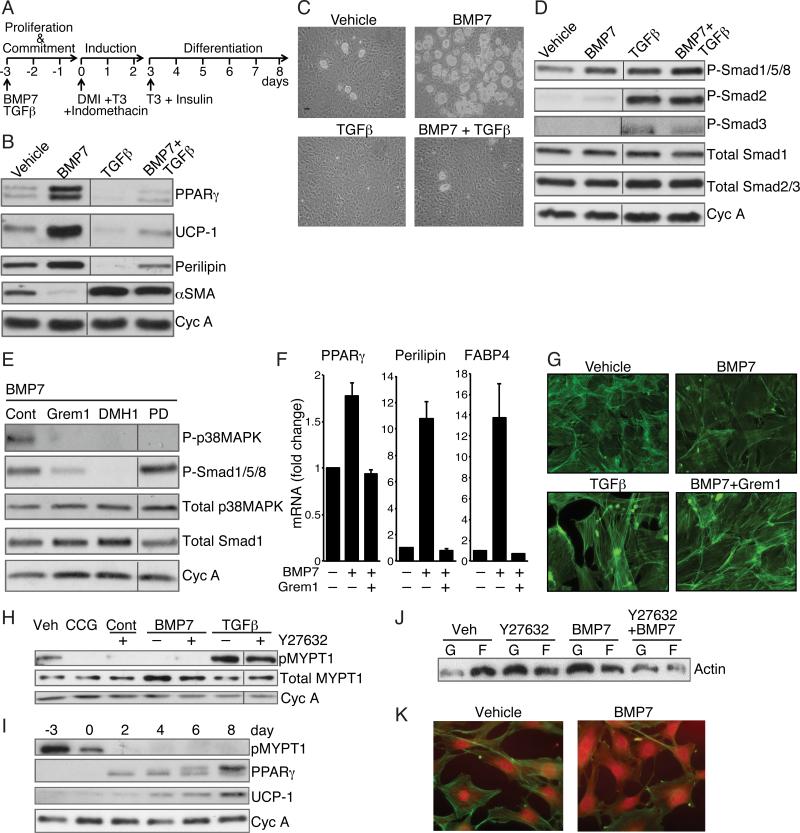
Figure 1. BMP7 and TGFβ Mediate Distinct Effects on Lineage Commitment of MSCs through alteration of ROCK activity.
(A) Differentiation protocol used for mesenchymal stem cells.
(B and C) Subconfluent C3H10T1/2 cells (B) or mouse bone marrow derived MSCs (Life Technologies, Gibco) (C) were exposed to BMP7 (6.3 nM) and/or TGFβ1 (1 nM) for 3 days, then induced to differentiate without BMP7 or TGFβ as in (A) Differentiation of cells was assessed by Western blot (B) or phase contrast microscopy (C).
(D and E) Western blot analysis of C3H10T1/2 cell proteins after a 10 min exposure to vehicle, BMP7, TGFβ or BMP7 and TGFβ (D) or BMP7 alone following a 2 h pretreatment with either 125 nM Gremlin1 or 200 nM Dorsmorphin homologue (DMH1) or 50 μM PD169316 (E).
(F) Bone marrow MSCs were induced to differentiate in response to BMP7 as in A in presence or absence of Gremlin1 and RNA was subjected to qPCR analysis.
(G) Subconfluent C3H10T1/2 MSCs were exposed to TGFβ1 (1 nM) alone or BMP7 (6.3 nM) with or without Gremlin1 for 48 h, then stained with Alexa Fluor 488 phalloidin and visualized at 40x magnification.
(H) Subconfluent C3H10T1/2 MSCs were exposed to BMP7 or TGFβ with or without Y27632 (10 μM) or CCG1423 (10 μM) for 2 h at which stage protein lysates were analyzed for phospho-MYPT1.
(I) Protein extracts from C3H10T1/2 MSCs at the indicated times of differentiation (as illustrated in 1A) were analyzed by Western blot.
(J and K) Subconfluent, proliferating C3H10T1/2 MSCs were treated with Y27632 with or without BMP7 for 48 h and levels of F-actin versus G-actin were analyzed by Western blot (J) and immunofluorescence (K). Non-adjacent lanes on western blots (B, D, E, H) are indicated by a vertical line. See also Figure S1.