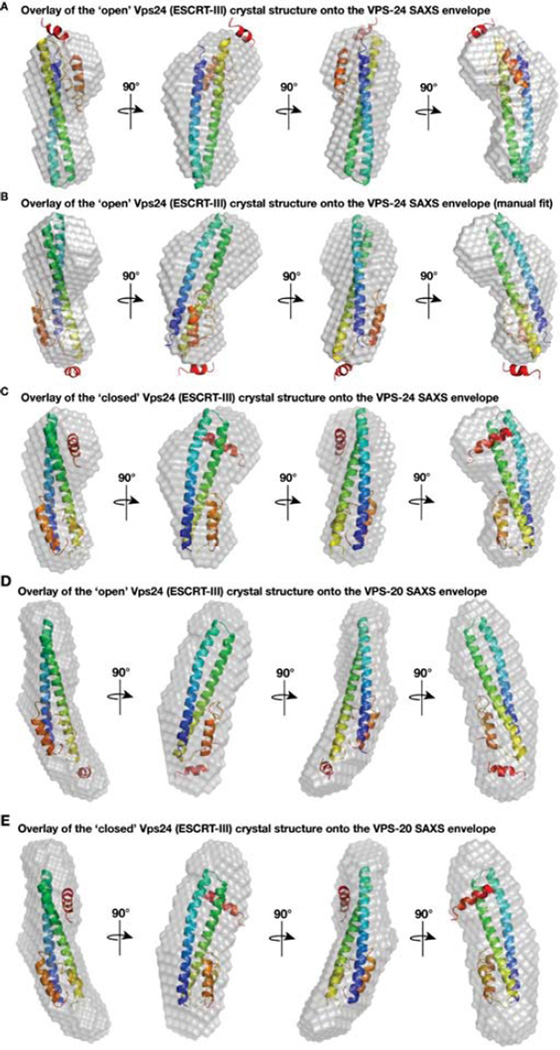
Figure 6. The closed human Vps24 crystal structure overlays well with the full length C. elegans VPS-24 SAXS envelope but not with the full length VPS-20 envelope.
(A) The program Supcomb20 was used to align one of the human Vps24 crystal structures (2GD5, representing its open conformation) with the SAXS model for C. elegans VPS-24, yielding a poor fit. (B) Manual refinement of the human Vps24 structure (2GD5) was performed in an attempt to optimize alignment with the SAXS model for C. elegans VPS-24. (C) The program Supcomb20 was used to align another human Vps24 structure (3FRT, representing the closed conformation) with the SAXS model for C. elegans VPS-24, which yields an excellent fit. (D) The program Supcomb20 was used to align one of the human Vps24 crystal structures (2GD5, representing its open conformation) with the SAXS model for full length VPS-20. (E) Alignment of another human Vps24 crystal structure (3FRT, representing its closed conformation) with the SAXS model for VPS-20 reveals a poor fit, with multiple regions of unaccounted density in the SAXS envelope.