Figure 4. An overview of PI3K inhibitors and their combination with other therapeutics.

(A) Molecular contexts dictating applications for isoform-selective PI3K inhibitors. Light orange boxes: Upregulation or mutation of receptor tyrosine kinases (RTKs), oncogenic RAS mutations, or activating p110α mutations all increase PtdIns(3,4,5)P3 production through p110α, which can be amplified by mutation or loss of PTEN. In these contexts use of p110α-selective inhibitors is effective. Blue boxes: In the absence of other oncogenic alterations, PTEN loss or mutation increases PtdIns(3,4,5)P3 production through p110β, perhaps due to RAC1- or CDC42-mediated p110β activation, or the basal activity of this isoform. In this context use of p110β-selective inhibitors is effective. Dark orange boxes: Upregulation or mutation of B cell receptors (BCRs), cytokine receptors, or other immune cell surface markers increases PtdIns(3,4,5)P3 production through p110δ. In this context use of p110δ-selective inhibitors is effective.
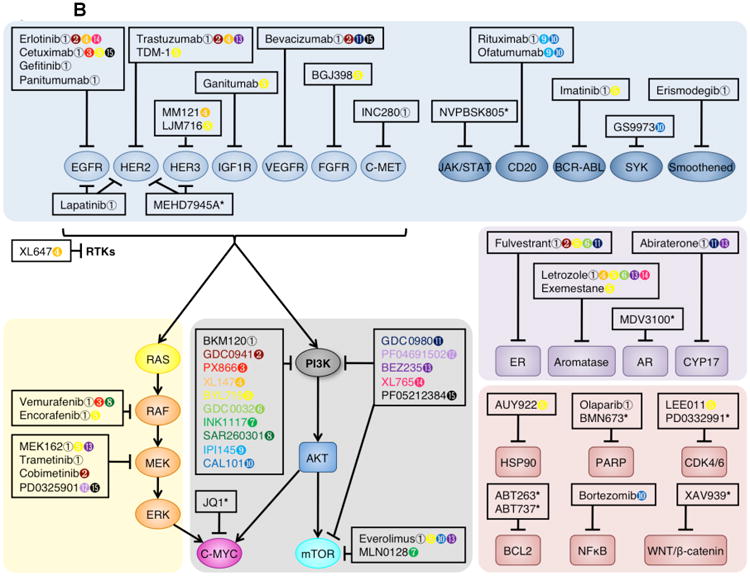
(B) Rational combination of PI3K inhibitors and other targeted therapeutics. Pan-PI3K and dual pan-PI3K and mTOR inhibitors are currently being tested in clinical trials (white box). These agents are being combined with mTOR-selective inhibitors (shown in dark orange), RAS-RAF-MEK-ERK pathway inhibitors (shown in light orange), RTK (shown in grey) or other membrane-associated protein inhibitors (shown in turquoise), hormone signaling inhibitors (shown in dark blue), and other agents inhibiting the cell cycle, apoptosis machinery, or other signaling pathways (shown in purple). Colored symbols indicate targeted therapeutics currently in clinical trials for combination with the designated PI3K inhibitor. For further detail, see Supplementary Table 3.
