Abstract
The ability of chemotherapeutic agents to induce apoptosis, predominantly via the mitochondrial (intrinsic) apoptotic pathway, is thought to be a major determinant of the sensitivity of a given cancer to treatment. Intrinsic apoptosis, regulated by the BCL-2 family, integrates diverse apoptotic signals to determine cell death commitment and then activates the nodal effector protein BAK to initiate the apoptotic cascade. In this study, we identified the tyrosine kinase BMX as a direct negative regulator of BAK function. BMX associates with BAK in viable cells and is the first kinase to phosphorylate the key tyrosine residue needed to maintain BAK in an inactive conformation. Importantly, elevated BMX expression prevents BAK activation in tumor cells treated with chemotherapeutic agents and is associated with increased resistance to apoptosis and decreased patient survival. Accordingly, BMX expression was elevated in prostate, breast and colon cancers compared to normal tissue, including in aggressive triple-negative breast cancers where BMX over-expression may be a novel biomarker. Furthermore, BMX silencing potentiated BAK activation rendering tumor cells hypersensitive to otherwise sublethal doses of clinically relevant chemotherapeutic agents. Our finding that BMX directly inhibits a core component of the intrinsic apoptosis machinery opens opportunities to improve the efficacy of existing chemotherapy by potentiating BAK-driven cell death in cancer cells.
Keywords: BAK, BMX, Apoptosis, Chemotherapy
Introduction
Resistance to apoptosis is one of the biggest barriers in developing effective cancer treatments. This is due, in part, to the fact that evading apoptosis is fundamentally required to allow cancer cells to survive, proliferate and disseminate and has been identified as one of the hallmarks traits of cancer described by Weinburg and Hannahan, (1, 2). Chemotherapeutic drugs, targeted at different cellular components that are common to all cells, remain a front-line treatments in cancer therapy and depend heavily on the intrinsic (mitochondrial) apoptotic pathway to elicit their effects (3). However, the ability of cancer cells to block or subdue the apoptotic machinery means that many chemotherapeutic agents that are currently used in clinic become less effective. The pleiotropic resistance often displayed by cancer cells of different origin to chemotherapeutic agents that damage cells in disparate ways, coupled with drug-related toxicities, pose major barriers to effective treatment (4, 5).
Death signals emanating from diverse stimuli such as stress, DNA damage, mitotic inhibitors or exposure to other cytotoxic agents are transduced through redundant signaling networks that ultimately converge on the mitochondrion to trigger mitochondrial outer membrane permeabilization (MOMP; 6, 7-9). Mitochondrial-driven apoptosis involving MOMP depends on the activation of BCL-2 effector proteins, BAK and BAX (9-12). The pre-dominance of death over survival signals determines whether MOMP takes place, a process involving oligomerization and pore formation by activated BAK/BAX that releases apoptogenic factors from the mitochondria and assists the dissipation of the mitochondrial transmembrane potential, a process that ultimately consigns a cell to death (13). BAK and BAX therefore exert their effects at a nodal commitment point in the apoptotic cascade. As yet, the signaling pathways that are subverted in cancer cells, which can confer a proliferative advantage by directly suppressing BAK activation, have not been delineated. We recently reported that in undamaged tumor cells BAK is present almost exclusively in a heavily phosphorylated form and, unlike BAX, requires dephosphorylation for activation (14-16). This discovery was potentially very significant because we hypothesized that elevated levels of a BAK-specific kinase, which may occur in cancer cells, would restrict BAK activation and limit cell killing in response to cytotoxic drugs. Importantly in this context, diverse apoptotic stimuli activate BAK, hence suppression of the first step required for the initiation of BAK activation would be predicted to suppress an apoptotic response to a wide variety of stimuli. Recalibrating the cellular apoptotic threshold by potentiating BAK-dependent cell killing through inhibition of kinase activity would be highly desirable, as this is likely to increase the efficacy of existing cytotoxic drugs or emerging targeted therapies.
There are many tumor types which are highly resistant to chemotherapy, such as triple negative breast cancers (TNBC) for which the overall prognosis is poor (17, 18). When TNBC recur, there is often little response to chemotherapy, and there are only a few treatment options in this setting. Thus, there is an urgent clinical need to identify new therapeutic targets in order to improve the outlook for these patients. Recent genetic profiling of these tumors reveals that there are diverse changes that occur during their development that makes identifying therapeutically targetable proteins complex (19). We hypothesize then that if a BAK kinase was overexpressed or up-regulated in these tumor types this would effectively enable the cell to block initiation of BAK-dependent apoptosis thereby increasing the apoptotic threshold of the tumor.
In this paper we identify BMX as the first tyrosine kinase that is able to modulate activation of BAK by direct phosphorylation of a key tyrosine residue, Y108. We find BMX expression to be up-regulated in numerous tumor types of major importance, including TNBC, and that BMX over-expression in tumor cells increased the apoptotic threshold of these cells. Furthermore, we demonstrate that modulation of BMX expression levels by RNAi can re-sensitize tumor cells to existing chemotherapeutic agents, by decreasing BAK phosphorylation resulting in increased BAK activation potential and enhanced levels of apoptotic cell death.
Materials and Methods
Cell Culture
HT1080, DU145, BT549 and MCF7 cells were obtained from and characterized by ATCC. HCT116bax−/−bak−/− cells were a gift from R. Youle. All cells were mycoplasma-free when tested with MycoAlert (Lonza) and used for less than 6 months of continuous passage.
Flow cytometric analysis of BAK conformation was determined using Ab-1 (AM03; Calbiochem) as previously described (20). Cytochrome c release was determined using clone 6H2.B4 (BD Biosciences (21)). Standard methods were used to determine Annexin V positivity.
RNA Interference
Standard methods were used for siRNA and shRNA transfections with details in supplementary Materials and Methods. Cells positive for shRNA constructs against BMX, PTPN21 and a control shRNA construct (pRS; Origene) were selected with 0.4 μg/ml puromycin.
Immunoprecipitation
Protein A/G agarose beads (Santa Cruz) and 1μg antibody as follows: pY108 BAK antibody previously characterized (16), PTPN21 (Abgent), BAK (BH3; Cell Signaling Technologies), BMX (C-17; Santa Cruz), pY100 (Cell Signaling) were used. Resultant samples were analyzed by immunoblotting.
Immunoblot analyses were carried out as previously described (16). Antibodies used were either previously published or listed (Supplementary materials and methods).
Proximity Ligation In Situ Assays were performed using a Duolink II Kit (Olink Bioscience) with anti–BMX (C-17; Santa Cruz) and BAK (BH3; Cell Signaling Technologies) antibodies.
In vitro kinase assay
BMX was isolated by immunoprecipation and incubated with GST, GST-WT BAK or GST-Y108A BAK protein substrates produced in E.coli and purified using standard techniques. Reactions were performed in kinase buffer containing 1.5 μCi [γ-33P]ATP and phosphorylated proteins detected by phosphoimage analysis (Typhoon analyzer). IP-Kinase assay was performed using untagged WT or Y108A BAK as a substrate and phosphorylated protein immunoprecipitated with pY108BAK antibody and magnetic protein G beads.
Tissue microarray and immunohistochemical analysis
Tissue microarrays were purchased from OCHRe and staining performed with ethical approval (Reference Number C02.216). Slides were immunostained, evaluated by an independent histopathologist and graded using a two-score system based on intensity score and proportion score as described previously (22), see supplementary material for further details.
Statistical analysis
All data are presented as mean ± SEM of three independent biologic experiments. T-tests were used to compare two experimental groups, differences were considered statistically significant where P <0.05.
Results
Identification of BMX as a potent inhibitor of BAK function
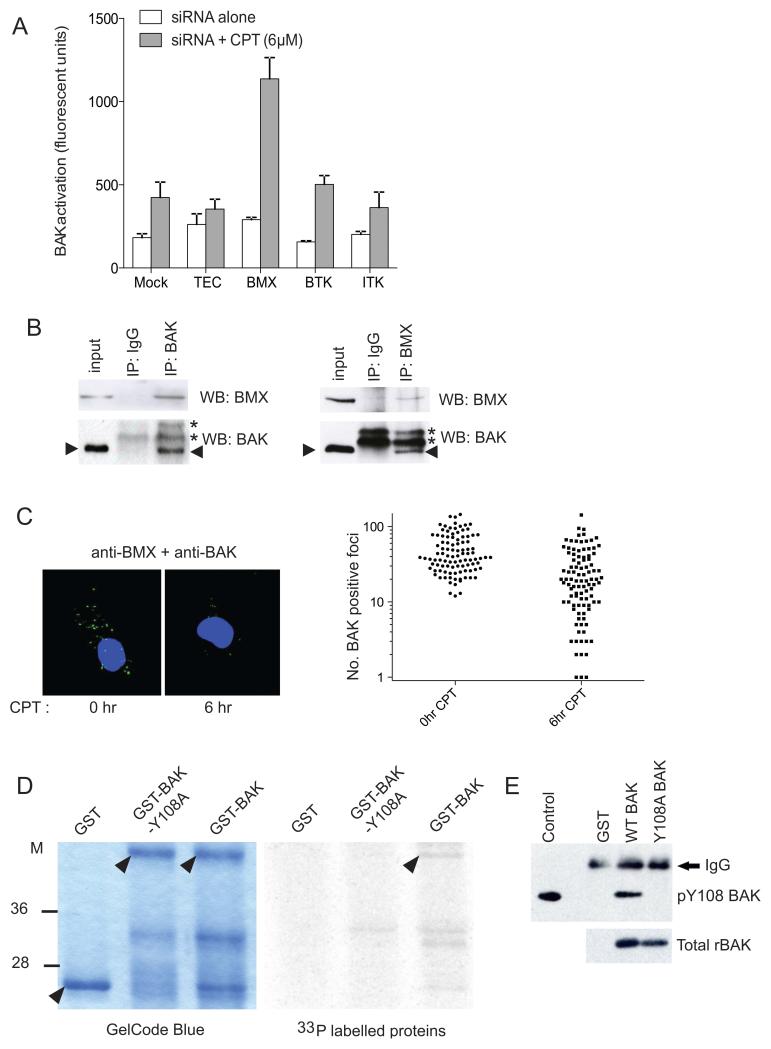
A conformational change at the N-terminal of BAK can be used to measure levels of BAK activation, but this can only occur if the phosphate at residue Y108 of BAK is first removed and BAK is rendered ‘activation competent’ (15, 16). We therefore wanted to identify kinases able to phosphorylate BAK at Y108 thus maintaining BAK in the inactive conformation. siRNA screening of the TEC tyrosine kinase family revealed that knockdown of BMX but not the other family members was able to significantly increase levels of BAK activation in response to camptothecin (CPT) treatment (Fig. 1A). To determine whether BMX was acting directly on BAK or via upstream signaling pathways we tested whether BMX could bind directly to BAK using co-immunoprecipitation/western blotting. The BMX-BAK complex could be immunoprecipitated using either a BAK or BMX antibody (Fig. 1B) indicating that the two proteins were found in a stable complex in healthy cells. The specificity of these co-immunoprecipitations was confirmed using BAK null and BMX knockdown cells as controls (supplementary Fig. S1). In contrast BAX was not found to co-immunoprecipitate with BMX (supplementary Fig. S2). To further examine the BMX-BAK complex in cells we used a proximity ligation in situ assay (PLISA) (23), which enables individual protein-protein complexes in cells to be studied. PLISA confirmed that in undamaged cells BMX was in close proximity to endogenous BAK (Fig. 1C) while treatment of cells for 6h with CPT caused a detectable decrease in the number of PLISA foci present in a significant number of the cells studied (Fig. 1C and supplementary Fig. S3A, B). Single color controls confirmed that both BAK and BMX levels were unaltered following treatment as both proteins could be detected at similar levels in both treated and untreated cells (supplementary Fig. S3C, D). These data suggest that BMX associates with BAK in undamaged cells, but following an apoptotic stimulus the BMX-BAK complex dissociates, which correlates with a decrease in pY108 BAK in cells following CPT treatment. The decrease in PLISA foci occurred before other morphological changes associated with apoptosis revealing that dissociation of the BMX-BAK complex is an early step in the BAK activation process, in line with the fast kinetics of BAK activation. To assess whether BMX was able to phosphorylate BAK at residue Y108, and was not just present in a complex with inactive BAK, we performed an in vitro kinase assay. BMX protein immunoprecipitated from HT1080 cells and incubated in the presence of [γ33P]ATP was able to phosphorylate purified GST-tagged WT, but not the Y108A mutant BAK recombinant protein (Fig. 1D). Furthermore, when the protein from the kinase assay was immunoprecipitated with an antiserum that specifically recognizes BAK that is phosphorylated at Y108, WT but not Y108A mutant BAK was pulled down (Fig. 1E). Together these assays show that BMX was able to phosphorylate BAK at this key regulatory residue. It is possible that there are other tyrosine kinases that are able to phosphorylate BAK which have yet to be identified, but we conclude that BMX is the first BAK tyrosine kinase identified to date.
Figure 1. BMX interacts with BAK and phosphorylates residue Y108.
A) FACS analysis of BAK activation in HT1080 cells following mock transfection (Mock) or siRNA knockdown of the indicated TEC kinases ± 4h CPT.
B) Co-immunoprecipitation of BMX-BAK complex from HT1080 cells using BAK or BMX antibody (arrowhead-BAK; *IgG bands).
C) Left panel: Representative images of PLISA in HT1080 cells. Green foci indicate BMX-BAK complexes ± 6h CPT treatment. Nuclei counter stained with DAPI. Right panel: Quantification of BMX-BAK complexes per cell following CPT treatment. For full microscope fields and negative controls see Supplementary Figure S2.
D) In vitro BMX kinase assay, BMX protein was immunoprecipitated from HT1080 cells (Supplementary Fig. S4) and activity on GST-tagged WT or Y108A BAK protein determined. Equal loading was confirmed by staining the gel with GelCode blue (arrowheads-Full length GST and GST-BAK proteins).
E) In vitro IP-Kinase assay were performed as D) using untagged WT or Y108A BAK as a substrate. Resultant phosphorylated protein detected by pY108BAK immunoprecipitation and western blotted for BAK. Equal loading was confirmed by western blotting of input substrate.
BMX is upregulated in Prostate, Breast and Colorectal Cancer
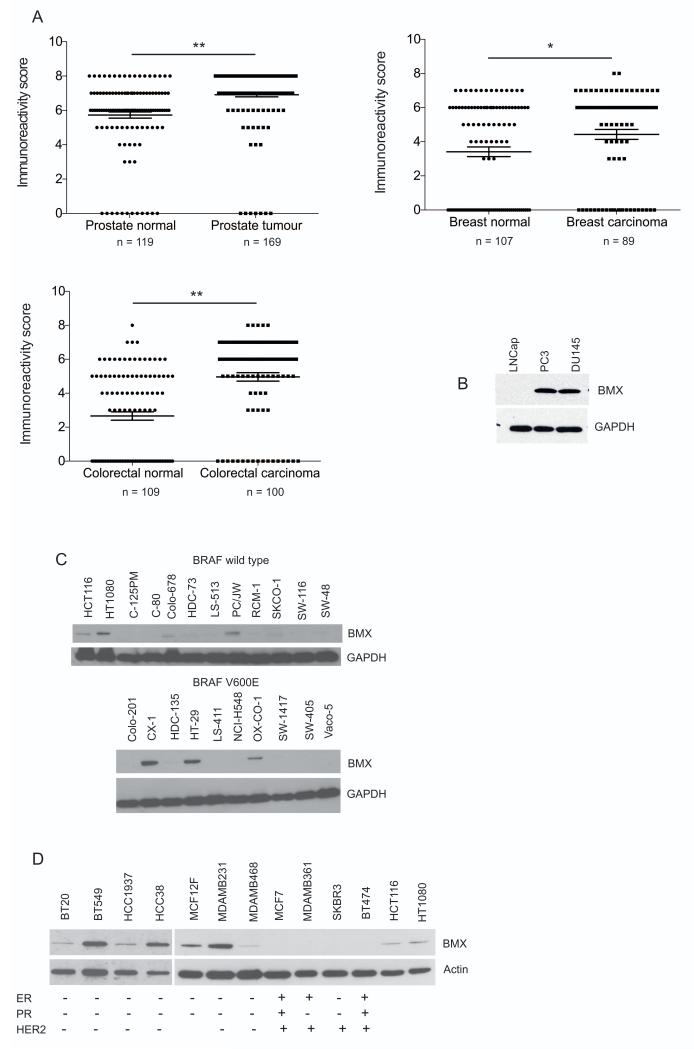
BMX is a member of the TEC family of non-receptor tyrosine kinases, which has been reported to have roles in modulating multiple cellular processes including proliferation, differentiation and apoptosis. Interestingly and supporting a role in pathogenesis elevated BMX levels in bladder cancer were reported to be associated with poorer prognosis (24), and BMX is important in the migration and invasion of breast cancer cells (25). Increased BMX expression has also been observed in neoplastic skin (26), renal cell carcinoma (27) and in prostate cancer (22, 28). To extend and explore these findings in greater depth we performed immunohistochemistry on tissue microarrays of cancer biopsies to determine BMX levels in a range of human tumor types. This analysis revealed that BMX levels were significantly elevated in prostate, breast and colon cancers when compared to normal tissue (Fig. 2A, supplementary Fig. S5). In conjunction with the immunohistochemistry data, western blotting of cell extracts from prostate cell lines revealed higher levels of BMX protein in PC3 and DU145 lines than in LNCaP cells (Fig. 2B). In a panel of colon cancer cell lines we found BMX over-expression was readily detectable in 5/20 cell lines investigated. BMX responds to receptor tyrosine kinase signaling that can be transduced through BRAF to MAP kinases, but we found no correlation between BMX levels and V600E BRAF mutation status (Fig. 2C). In a drive to identify individuals most at risk of developing the disease, genome wide analysis of mutations and SNPs in breast cancers have revealed a complex tumor landscape and identified many new cancer-associated genes and loci, however no activating or other mutations in BMX were reported (29-32). So-called triple-negative breast cancers (TNBC) are characterized by a lack of expression of three important receptors; estrogen, progesterone and HER2, and display heterogeneity in mutation patterns. TNBC accounts for ~15% of all breast cancers at diagnosis, but the aggressive nature of the tumors and poor prognosis likely accounts for a disproportionate share in mortality rates (33). Importantly, we found that 6/6 TNBC lines investigated had much higher levels of BMX when compared to cell lines in which at least one receptor was expressed and lacked any detectable BMX (Fig. 2D). Thus, while the acquisition of multiple mutations that in combination can alter the activity of the many diverse signaling pathways upstream of BMX activity, in some tumors over-expression of BMX may promote evasion of apoptosis and help confer a survival advantage to cancer cells. However, the mechanism by which BMX is able to achieve this by modulating cellular apoptosis to promote cancer cell survival has not been identified to date. Importantly, our data suggests that the anti-apoptotic role of BMX is largely through its regulation of BAK phosphorylation at Y108.
Figure 2. BMX expression is upregulated in a panel of tumors and cell lines.
A) Tissue microarray arrays were stained with anti-BMX antibody and quantification of staining in normal versus cancer samples shown in scatter plots. Difference between normal versus cancer samples was determined by t-test * p<0.05, **p<0.0001.
B) Western blot of BMX in prostate cancer cell lines. GAPDH used as a loading control.
C) Western blot of BMX in colon cancer cell lines with either wild type BRAF (top panel) or V600E mutant BRAF (bottom panel). GAPDH used as a loading control.
D) Western blot of BMX in breast cancer cell lines with known receptor status, estrogen receptor (ER), progesterone receptor (PR) or HER2. Actin used as a loading control.
BMX requires PTPN21 for BAK phosphorylation
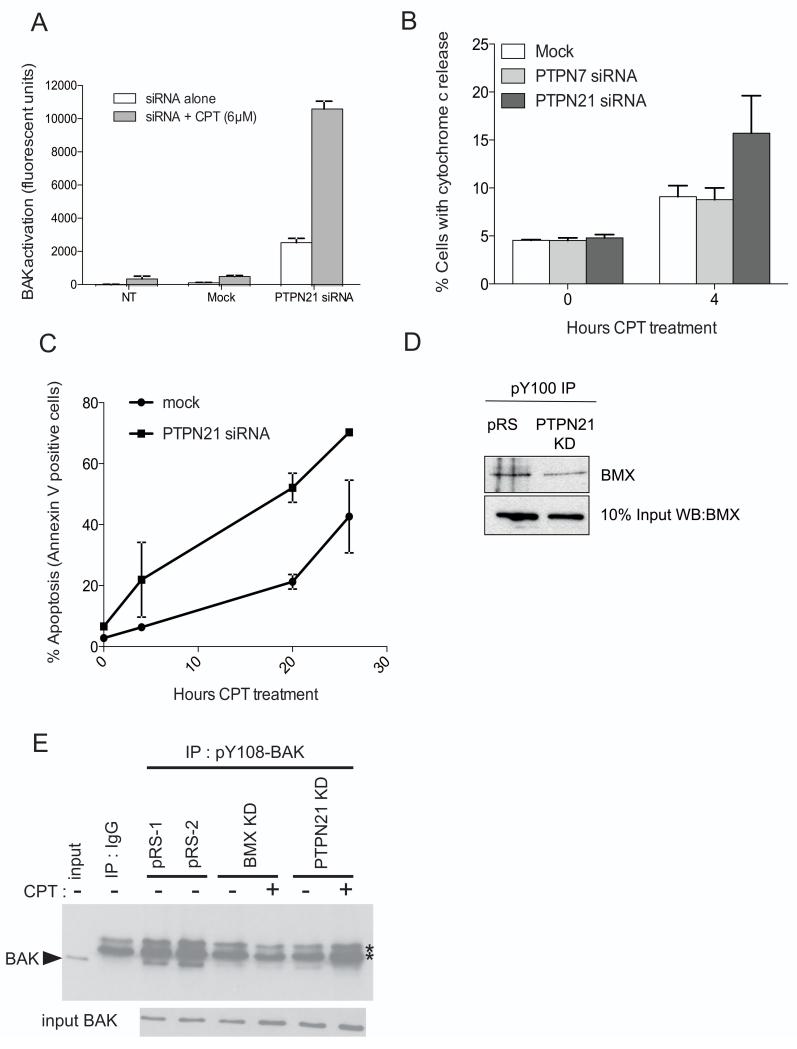
Activation of BMX requires both trans-phosphorylation and auto-phosphorylation. Once these events have occurred a substantial increase in kinase activity is observed (34). In addition, non-receptor protein tyrosine phosphatase 21 (PTPN21) has been reported to bind to and regulate the activity of BMX (35), indeed in HT1080 cells BMX can be immunoprecipitated with PTPN21 (supplementary Fig. S6). Structure-function studies of PTPN21 performed using the phosphatase domain failed to identify any substrate motifs (36), but the modulation of Src and EGF signaling pathways suggests that the full-length protein may be active (37). Previous siRNA screening of the non-receptor protein tyrosine phosphatase family to identify phosphatases involved in BAK activation revealed that knockdown of some phosphatases led to a modest increase in BAK activation (16). However, further investigations indicated that knockdown of PTPN21 both by siRNA and shRNA (knockdown determined by qRT-PCR supplementary Fig. S7) also increased the basal levels of BAK in a conformationally active state even in the absence of any damage signal, presumably due to an increase in the number of under-phosphorylated BAK molecules. However, a dramatic potentiation of BAK activation occurred when an apoptotic signal was induced using a chemotherapeutic DNA damaging agents such as CPT (Fig. 3A and supplementary Fig. S8B), or UV radiation (supplementary Fig. S8A). Concomitant with the increase in levels of active BAK, we also found an increase in the number of cells showing cytochrome c release early after CPT treatment (Fig. 3B) and actively undergoing apoptosis as judged by Annexin V positive staining (Fig. 3C and supplementary Fig.S9) when PTPN21 was silenced - as compared to PTPN7 which we reported previously to have no effect on BAK activation (16). These results at first appear counterintuitive as such an observed increase in BAK activation would be predicted to be due to the relief from inhibition of BMX kinase activity that suppressed the initial step of BAK activation through phosphorylation at Y108. Measurement of the level of BMX auto-phosphorylation, a surrogate marker of BMX activity, by phospho-tyrosine IP-western blotting showed that PTPN21 knockdown decreased the activity of BMX (Fig. 3D). This finding suggested that BMX acts in concert with, and may require PTPN21 to direct its activity to BAK. It is therefore the role of PTPN21 in regulating BMX activity, rather than any direct activity on BAK itself that is important, implying that the PTPN21-BMX axis is key in determining the level of BAK phosphorylation at Y108. To test this, we performed IP-western blots with an antiserum that specifically recognizes BAK that is phosphorylated at Y108. While pY108-BAK was readily detectable in undamaged cells, we found that siRNA knockdown of either PTPN21 or BMX each resulted in markedly decreased levels of pY108-BAK regardless of the presence of DNA damage (Fig. 3E, supplementary Fig. S10). We conclude that BAK is a novel target of BMX signaling and that BMX is primarily responsible for maintaining pY108-BAK phosphorylation in these cells; however BMX requires PTPN21 for this activity as knockdown of either PTPN21 or BMX leads to BAK under-phosphorylation, enabling a potentiation of BAK activation and cell killing.
Figure 3. PTPN21-BMX is involved in regulation of BAK Y108 phosphorylation and increased sensitivity to apoptosis.
A) FACS analysis of BAK activation in HT1080 cells with either no-transfection (NT), mock transfection (Mock) or siRNA knockdown of PTPN21 ± 4h CPT.
B) FACS analysis of cells with cytochrome c release in HT1080 cells with PTPN7 or PTPN21 siRNA knockdown ± 4h CPT treatment.
C) Mean percentage of Annexin V positive HT1080 cells 48h post siRNA knockdown of PTPN21 over a 24 hour time course of CPT treatment.
D) Immunoprecipitation of pBMX from HT1080 cells expressing empty vector shRNA (pRS) or shRNA against PTPN21 using pan-tyrosine antibody pY100, then western blotted for BMX.
E) Immunoprecipitation of pY108-BAK from HT1080 cells expressing empty vector shRNA (pRS), or shRNA against PTPN21 or BMX, followed by western blotting for BAK (arrowhead); * indicates IgG bands.
BMX overexpression raises the apoptotic threshold of cancer cell lines
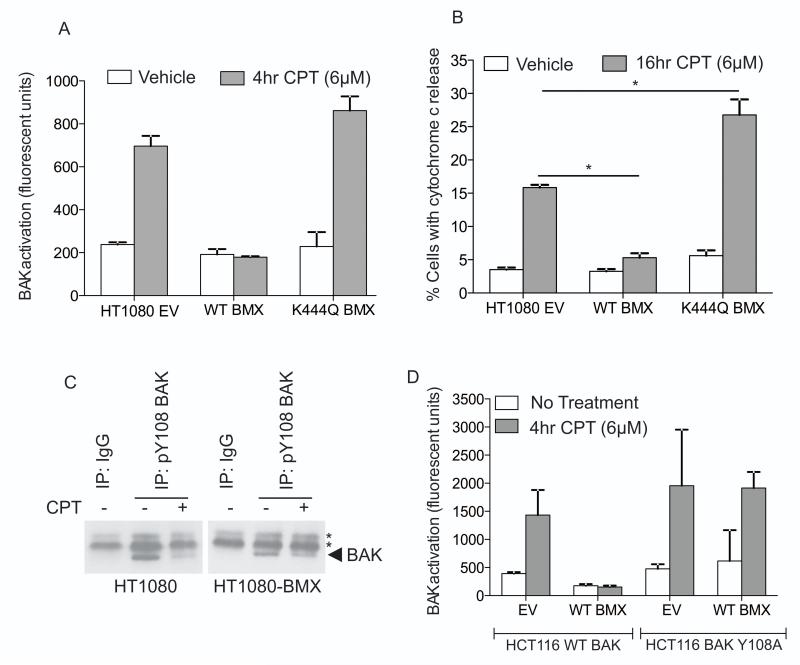
To define further the link between BMX and BAK and simulate the effects of BMX overexpression in cancers, we developed a BMX over-expression cell line model in which we reasoned that the increased BMX levels would suppress BAK activation following CPT treatment. Transfection of HT1080 cells with plasmids expressing BMX showed that while expression of the wild-type protein was capable of suppressing BAK activation compared to cells transfected with the pEFIRES vector (38), expression of a previously characterized kinase dead BMX mutant (K444Q) (34) had no effect on BAK activation following CPT treatment (Fig. 4A). In line with this finding, over-expression of wild-type BMX also inhibited the release of cytochrome c from cells treated with CPT. Expression of the BMX K444Q mutant not only failed to suppress cytochrome c release following CPT treatment, but instead resulted in a significant increase in cytochrome c release suggesting either that this mutant acted in a dominant negative manner, or that the activity of other cellular targets that also impinge upon apoptotic signaling might have been altered (Fig. 4B). Increasing levels of active BMX in HT1080 cells also increased the cellular IC50 value ~4 fold as determined by MTT assay in response to CPT from 0.004 μM to 0.0165 μM, whereas in comparison, cells expressing the K444Q BMX mutant had an IC50 of 0.005 μM (that was not significantly different from control cells transfected with the pEFIRES vector). Consistent with these findings, HT1080 cells over-expressing BMX showed only a small decrease in phosphorylation of Y108 following CPT treatment (Fig. 4C, supplementary Fig. S11). Together these findings indicate that cells with increased levels of BMX maintain BAK phosphorylation at Y108 and cannot initiate apoptosis, thereby conferring increased resistance to cytotoxic agents. To further confirm that the BMX activity on BAK is the key determinant for the observed increase in resistance to CPT we similarly over-expressed BMX in HCT116bax−/−,bak−/− cells that have been reconstituted to express either WT or Y108A mutant BAK. Over-expression of BMX was able to block BAK activation in response to CPT treatment, as measured by N-terminal BAK conformational change, but only in cells expressing wild-type (WT) but not the Y108A BAK mutant (Fig. 4D).
Figure 4. BMX over-expression suppresses BAK activation.
A) FACS analysis of BAK activation in HT1080 cells expressing empty vector (EV), wild-type (WT) BMX or the kinase dead K444Q mutant.
B) FACS analysis of cells with cytochrome c release in HT1080 cell lines over-expressing WT or K444Q BMX ± 16h CPT treatment * p<0.05.
C) Immunoprecipitation of phospho-Y108BAK from HT1080 or HT1080 cells over-expressing BMX ± 4h CPT treatment (arrow indicates BAK).
D) FACS analysis of BAK activation in HCT116 cells expressing WT or Y108A BAK following overexpressing WT BMX or EV where indicated ± 4h CPT treatment.
Knockdown of BMX lowers the apoptotic threshold by increasing BAK activation
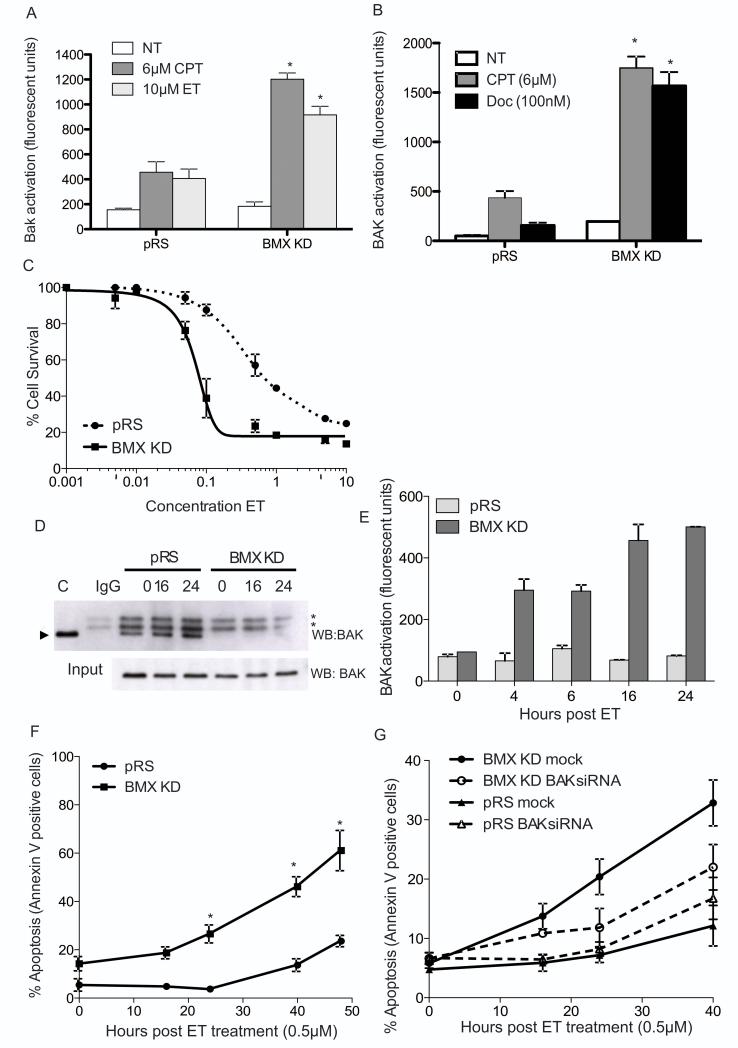
Following on from the reported roles of BMX in the pathogenesis of numerous cancers types there has been increasing interest in developing BMX inhibitors (Reviewed in 39). Therefore, to evaluate the effect of BMX inhibition on BAK activation we developed cell lines in which BMX was stably knocked down by shRNA. As predicted knockdown of BMX alone modulated pY108 BAK levels (Fig. 3E) but did not cause BAK activation (Fig. 5A). However, knockdown of BMX in HT1080 cells significantly increased the amount of BAK that underwent activation, as measured by BAK N-terminal conformational change, in response to treatment with CPT or Etoposide (ET) compared to empty vector controls (Fig. 5A). Levels of BAX conformational change, however, were not effect by BMX knockdown (supplementary Fig. S12). Similar potentiation of BAK activation by BMX knockdown was seen in DU145 prostate cancer cells (supplementary Fig.S13B) treated with docetaxel, an important therapeutic agent currently in widespread clinical use (Fig. 5B, supplementary Fig. S13A), and the in breast cancer cell lines BT549 and MCF7 which have high and low/undetectable levels of BMX respectively (supplmentary Fig. S14). Overall, the magnitude of the sensitization observed did correlate with the levels of BMX in the cells, suggesting that BMX may be an excellent biomarker of cells that are employing BMX overexpression as a mechanism to evade apoptosis. The increase in BAK activation in HT1080 cells correlated with a decrease in cellular IC50 values as determined by MTT assay (Supplementary Table 1), most strikingly in response to ET treatment where a ~10-fold increase in sensitivity was observed in cells with BMX levels knocked down compared to control cells (Fig. 5C). This suggested that a dose of ET that was non-toxic to the control cells would elicit cell death in BMX knockdown cells, highlighting the huge potential the use of a BMX inhibitor could have in combination with existing chemotherapeutic agents. To test this, BMX- silenced cells were treated with 0.5 μM ET, a dose that caused minimal cell killing in control cells but caused significant levels of death in cells with silenced BMX. Examination of the effects this dose of ET had on BAK activation, revealed that 0.5 μM ET was insufficient to trigger BAK dephosphorylation (Fig. 5D) or N-terminal conformational change in the control cells (Fig. 5E). Whereas, in the BMX knockdown cells, where pY108 BAK levels were already significantly decreased (Fig. 5D&E), BAK activation was readily detectable as early as 4 hours following ET treatment and continued to increase with time (Fig. 5E), which correlated with the increase in apoptotic cells observed by Annexin V staining at the same time points (Fig. 5F). At later time points, a small increase in Annexin V positive cells was apparent in the BMX knockdown cells that may reflect the loss of other pro-survival activities of BMX unrelated to BAK. To confirm that the difference in apoptosis observed in the BMX knockdown cells was BAK-dependent, transient knockdown of BAK expression by siRNA in BMX knockdown cells partially reversed the sensitization to low dose ET, indicating that the increase in sensitivity observed in this cell system was predominantly caused by the reduced activity of BMX on BAK (Fig. 5G).
Figure 5. BMX knockdown promotes BAK activation and cell killing.
A) FACS analysis of BAK activation in HT1080 cells expressing BMX shRNA or empty vector (pRS) ± 4h CPT or ET treatment *p<0.05.
B) FACS analysis of BAK activation in DU145 prostate cancer cells expressing BMX shRNA or empty vector (pRS) ± 4h CPT or docetaxel (DOC) treatment *p<0.05.
C) Mean cell survival of HT1080 cells with BMX knockdown as determined by MTT assay following 96hr exposure to a range of ET concentrations as indicated.
D) Immunoprecipitation of pY108-BAK in HT1080 control and BMX knockdown cells at 0, 16 and 24h +/− low dose ET (0.5 μM) treatment (arrow indicates BAK).
E) FACS analysis of BAK activation of HT1080 control and BMX knockdown cells treated in part D of figure over 24h time course of low dose ET treatment.
F) Mean percentage of HT1080 BMX knockdown cells over a 48 hour time course of low dose ET treatment.
G) Mean percentage of Annexin V positive HT1080 BMX knockdown cells over a 40 hour time course of low dose ET treatment, 48 hours post siRNA knockdown of BAK.
Discussion
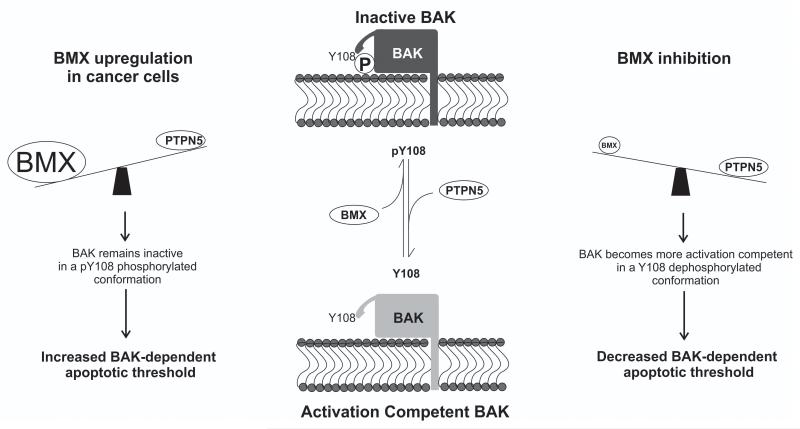
In this study we investigated the molecular mechanism through which the activity of the apoptotic machinery can be suppressed in cancer cells. Chemotherapeutic drugs remain a main stay of front-line clinical cancer therapy. Improving the efficacy of treatments, or overcoming acquired resistance, are priorities as they can have a major impact on disease progression and overall survival rates. We demonstrate for the first time here, that BMX, acting in concert with PTPN21, phosphorylates the apoptotic regulator BAK, thereby suppressing BAK activation to favor survival of cells exposed to cytotoxic agents. These findings define a novel mechanism that helps to explain how cancer cells can manifest increased resistance to multiple chemotherapeutic drugs that damage cells in different ways. The initial conversion of BAK from a Y108-phosphorylated to Y108-dephosphorylated form is the key switch that enables BAK activation to proceed and cause MOMP (16). We now propose that the size of the pool of Y108-dephosphorylated BAK will determine a given cells sensitivity to undergo apoptosis, therefore setting the apoptotic threshold within that cell (Fig. 6). Up-regulation of BMX expression, as observed in many cancer types and cancer cell lines (Fig. 2), maintains BAK in the inactive Y108 phosphorylated form, which in turn enables cancer cells to suppress BAK-driven cell death in response to diverse agents by modulating a single nodal point that integrates survival versus death signaling in the apoptotic cascade.
Figure 6. Model of proposed mechanism by which BAK-dependent apoptotic threshold is determined.
The pool of Y108 dephosphorylated ‘activation competent’ BAK available for activation is dependent on the balance between the activity of PTPN5 (16) and BMX.
BMX has been implicated in the development of prostate cancer (22, 40), where elevated levels may compensate for androgen deprivation (28, 41). Further, a requirement of BMX for glioblastoma stem cell survival has been identified (42), as well as a role for BMX in the pathogenesis of bladder cancer (24). At a molecular level, BMX has been shown to be involved in controlling a number of important pathways downstream of PI3K signaling (43, 44), including cell proliferation involving FAK and PAK1 to promote the migration and invasion of breast cancer cells (25). Furthermore, there are numerous reports that modulation of BMX expression levels is able to influence cellular apoptosis (45, 46); however, to date no BMX substrates directly involved in the process have been identified. BMX is known to be activated by signaling pathways known to promote cell proliferation and survival (47), therefore its role in phosphorylating Y108-BAK that we now propose maintains BAK in an inactive conformation thereby providing another level of regulation that helps to push the life death cellular balance in the direction of survival. Our findings therefore, reveal a mechanism through which BMX plays a major role in conferring apoptotic resistance to cancer cells, a function that underpins other pathogenic activities associated with BMX over-expression.
A clearer understanding of the mutational processes that drive cancer cells and how they impact on complex signaling networks that confer survival and growth advantage is ultimately needed. Genome sequencing has emerged as a powerful tool holding the exciting prospect of identifying genes and pathways altered in cancer cells that may provide new targets for intervention. However, BMX and BAK have been found to be mutated in only 0.51% and 0.095% respectively of the total samples analyzed (COSMIC database). Our findings raise the important prediction that malignancies may still be driven by tyrosine kinase signaling even though dominant mutations have not been identified. We propose a paradigm in which signaling pathways subverted through genetic and epigenetic alterations can impact directly on and suppress the activity of the otherwise intact apoptotic machinery. Modulation of a single nodal point in the apoptotic machinery, as exemplified by the phospho-regulation of BAK, enables diverse signaling pathways to converge and produce a common phenotypic outcome that favors survival of cancer cells.
With increasing interest in developing specific BMX inhibitors (Reviewed in 39) and the expansion of clinical trials of existing agents shown to have activity against BMX, for example EGFR inhibitors (48), it would be interesting to investigate the effects of these agents on the BAK activation pathway. Our study suggests that, together with any stand-alone activity, these agents may have, they also have the potential to lower the apoptotic threshold of tumor cells. We present evidence that BMX inhibition presents an attractive novel approach to potentiate BAK-driven cell killing in appropriate combination with cytotoxic drugs. Such combination strategies could be employed to both reverse the apoptotic resistance associated with cancers and render tumor cells susceptible to killing by otherwise sub-lethal doses of drug, by increasing the size of the Y1080-dephposphorylated BAK pool within the cell. A further significant clinical implication of our results is that we can identify specific cancer cell types for which BMX may be an important biomarker for survival dependency.
In summary, we establish that BMX is the first kinase identified to phosphorylate the apoptotic regulator BAK. Consistent with published data we find BMX expression to be up-regulated in numerous cancer types and demonstrate that up-regulation of BMX maintains BAK phosphorylation, thereby enabling the cancer cells to suppress BAK-driven apoptosis in response to diverse stimuli. Furthermore, reduction of BMX by RNAi significantly lowered the apoptotic threshold of tumor cells rendering them much more sensitive to chemotherapeutic agents. Finally, we propose that BMX expression could be used as a biomarker to identify patients in which inhibition of BMX could be used as a mechanism to lower the apoptotic threshold of cells to increase the efficacy of existing cytotoxic agents.
Supplementary Material
Acknowledgments
We thank Prof Peter McHugh and Prof. Marion MacFarlane for reading drafts of the manuscript; Dr Richard Youle for kind gift of HCT116bax−/−bak−/− double knockout cells; Leticia Campo for help with staining and Dr David Delaney and Dr Francesco Pezzella for scoring the TMAs; Sir Walter Bodmer for the colon cancer cell lines and Carolin Schmidke for assistance with western blots; members of the WIMM FACS core facility; MRC Toxicology Unit for reagents and support. Funding was from Cancer Research UK.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 4.Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends in cell biology. 2001;11:S22–6. doi: 10.1016/s0962-8924(01)02124-9. [DOI] [PubMed] [Google Scholar]
- 5.Makin G, Hickman JA. Apoptosis and cancer chemotherapy. Cell and tissue research. 2000;301:143–52. doi: 10.1007/s004419900160. [DOI] [PubMed] [Google Scholar]
- 6.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L, Morselli E, Kepp O, Vitale I, Rigoni A, Vacchelli E, et al. Mitochondrial gateways to cancer. Molecular aspects of medicine. 2010;31:1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 9.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 10.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llambi F, Green DR. Apoptosis and oncogenesis: give and take in the BCL-2 family. Current opinion in genetics & development. 2011;21:12–20. doi: 10.1016/j.gde.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–31. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azad A, Fox J, Leverrier S, Storey A. Blockade of the BAK hydrophobic groove by inhibitory phosphorylation regulates commitment to apoptosis. PLoS One. 2012;7:e49601. doi: 10.1371/journal.pone.0049601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J, Azad A, Ismail F, Storey A. “Licensed to kill”: tyrosine dephosphorylation and Bak activation. Cell Cycle. 2011;10:598–603. doi: 10.4161/cc.10.4.14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JL, Ismail F, Azad A, Ternette N, Leverrier S, Edelmann MJ, et al. Tyrosine dephosphorylation is required for Bak activation in apoptosis. EMBO J. 2010;29:3853–68. doi: 10.1038/emboj.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 18.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, et al. Cell damage-induced conformational changes of the proapoptotic protein Bak in vivo precede the onset of apoptosis. The Journal of cell biology. 1999;144:903–14. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterhouse NJ, Trapani JA. A new quantitative assay for cytochrome c release in apoptotic cells. Cell Death Differ. 2003;10:853–5. doi: 10.1038/sj.cdd.4401263. [DOI] [PubMed] [Google Scholar]
- 22.Dai B, Kim O, Xie Y, Guo Z, Xu K, Wang B, et al. Tyrosine kinase Etk/BMX is up-regulated in human prostate cancer and its overexpression induces prostate intraepithelial neoplasia in mouse. Cancer Res. 2006;66:8058–64. doi: 10.1158/0008-5472.CAN-06-1364. [DOI] [PubMed] [Google Scholar]
- 23.Jarvius M, Paulsson J, Weibrecht I, Leuchowius KJ, Andersson AC, Wahlby C, et al. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol Cell Proteomics. 2007;6:1500–9. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Sun F, Guo Z, Li W, Alfano A, Chen H, et al. Tyrosine kinase ETK/BMX is up-regulated in bladder cancer and predicts poor prognosis in patients with cystectomy. PLoS One. 2011;6:e17778. doi: 10.1371/journal.pone.0017778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, et al. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J Biol Chem. 2001;276:29403–9. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 26.Paavonen K, Ekman N, Wirzenius M, Rajantie I, Poutanen M, Alitalo K. Bmx tyrosine kinase transgene induces skin hyperplasia, inflammatory angiogenesis, and accelerated wound healing. Mol Biol Cell. 2004;15:4226–33. doi: 10.1091/mbc.E04-03-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuang J, Tu X, Cao K, Guo S, Mao X, Pan J, et al. The expression and role of tyrosine kinase ETK/BMX in renal cell carcinoma. J Exp Clin Cancer Res. 2014;33:25. doi: 10.1186/1756-9966-33-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai B, Chen H, Guo S, Yang X, Linn DE, Sun F, et al. Compensatory upregulation of tyrosine kinase Etk/BMX in response to androgen deprivation promotes castration-resistant growth of prostate cancer cells. Cancer Res. 2010;70:5587–96. doi: 10.1158/0008-5472.CAN-09-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michailidou Kyriaki, Hall Per, Gonzalez-Neira Anna, Ghoussaini Maya, Dennis Joe, Milne Roger I, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nature Genetics. 2013;45:353–61. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Laurentiis M, Cianniello D, Caputo R, Stanzione B, Arpino G, Cinieri S, et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer treatment reviews. 2010;36(Suppl 3):S80–6. doi: 10.1016/S0305-7372(10)70025-6. [DOI] [PubMed] [Google Scholar]
- 34.Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, et al. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Molecular and cellular biology. 2000;20:2043–54. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jui HY, Tseng RJ, Wen X, Fang HI, Huang LM, Chen KY, et al. Protein-tyrosine phosphatase D1, a potential regulator and effector for Tec family kinases. The Journal of biological chemistry. 2000;275:41124–32. doi: 10.1074/jbc.M007772200. [DOI] [PubMed] [Google Scholar]
- 36.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–63. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardone L, Carlucci A, Affaitati A, Livigni A, DeCristofaro T, Garbi C, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol. 2004;24:4613–26. doi: 10.1128/MCB.24.11.4613-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobbs S, Jitrapakdee S, Wallace JC. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochemical and biophysical research communications. 1998;252:368–72. doi: 10.1006/bbrc.1998.9646. [DOI] [PubMed] [Google Scholar]
- 39.Jarboe JS, Dutta S, Velu SE, Willey CD. Mini-review: bmx kinase inhibitors for cancer therapy. Recent Pat Anticancer Drug Discov. 2013;8:228–38. doi: 10.2174/15748928113089990043. [DOI] [PubMed] [Google Scholar]
- 40.Xue LY, Qiu Y, He J, Kung HJ, Oleinick NL. Etk/Bmx, a PH-domain containing tyrosine kinase, protects prostate cancer cells from apoptosis induced by photodynamic therapy or thapsigargin. Oncogene. 1999;18:3391–8. doi: 10.1038/sj.onc.1202687. [DOI] [PubMed] [Google Scholar]
- 41.Kim O, Jiang T, Xie Y, Guo Z, Chen H, Qiu Y. Synergism of cytoplasmic kinases in IL6-induced ligand-independent activation of androgen receptor in prostate cancer cells. Oncogene. 2004;23:1838–44. doi: 10.1038/sj.onc.1207304. [DOI] [PubMed] [Google Scholar]
- 42.Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang X, Borgesi RA, McKnight NC, Kaur R, Carpenter CL, Balk SP. Activation of nonreceptor tyrosine kinase Bmx/Etk mediated by phosphoinositide 3-kinase, epidermal growth factor receptor, and ErbB3 in prostate cancer cells. The Journal of biological chemistry. 2007;282:32689–98. doi: 10.1074/jbc.M703412200. [DOI] [PubMed] [Google Scholar]
- 44.Vogt PK, Hart JR. PI3K and STAT3: a New Alliance. Cancer Discov. 2011;1:481–86. doi: 10.1158/2159-8290.CD-11-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo L, Zhou Y, Sun Y, Zhang F. Non-receptor tyrosine kinase Etk regulation of drug resistance in small-cell lung cancer. European journal of cancer. 2010;46:636–41. doi: 10.1016/j.ejca.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Zhu W, Zhang J, Guo L. Tyrosine kinase Etk/BMX protects nasopharyngeal carcinoma cells from apoptosis induced by radiation. Cancer biology & therapy. 2011;11:690–8. doi: 10.4161/cbt.11.7.15060. [DOI] [PubMed] [Google Scholar]
- 47.Chau CH, Chen KY, Deng HT, Kim KJ, Hosoya K, Terasaki T, et al. Coordinating Etk/Bmx activation and VEGF upregulation to promote cell survival and proliferation. Oncogene. 2002;21:8817–29. doi: 10.1038/sj.onc.1206032. [DOI] [PubMed] [Google Scholar]
- 48.Hur W, Velentza A, Kim S, Flatauer L, Jiang X, Valente D, et al. Clinical stage EGFR inhibitors irreversibly alkylate Bmx kinase. Bioorg Med Chem Lett. 2008;18:5916–9. doi: 10.1016/j.bmcl.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.