Table 2.
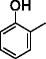
Propargylic substitution reactions of 1,3-diphenyl-2-propyn-1-ol with various nucleophilic compounds using PMA@MSS@FeCo/GC a

| |||
|---|---|---|---|
| Entry | Substrate | Product | Conversion (%) b |
| 1 |
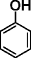
|
|
100 |
| 2 |

|
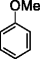
|
99 |
| 3 |

|

|
94 |
| 4 |

|

|
75 |
| 5 |

|

|
67 |
| 6 |

|
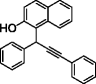
|
93 |
| 7 |

|

|
76 |
| 8 |
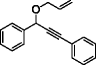
|

|
31 |
| 9 |
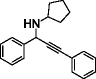
|

|
79 |
aReaction conditions: 1,3-diphenyl-2-propyn-1-ol (0.19 ml, 1.0 mmol), nucleophile (1.2 mmol), acetonitrile (5.0 mL), catalyst (0.05 mol%), and time ( 30 min). bDetermined by 1H NMR spectroscopy. Yields are based on the amount of propargylic alcohol.
