Abstract
Background
Innovations in biologics offer great promise in the treatment of patients with orthopaedic conditions and in advancing our ability to monitor underlying disease pathophysiology. Our understanding of the pathophysiology of hip osteoarthritis (OA) has improved significantly in the last decade. Femoroacetabular impingement (FAI) and hip dysplasia are increasingly recognized and treated as forms of prearthritic hip disease, yet the inability of radiographic and MR imaging to identify patients before the onset of irreversible articular cartilage injury limits their use for early diagnosis and treatment of patients with these conditions. Molecular biomarkers, as objectively measureable indicators of the pathophysiology of hip OA, have the potential to improve diagnosis, disease staging, and prognosis of hip OA and prearthritic hip disease. Although research into molecular biomarkers of hip OA has been conducted, investigations in prearthritic hip disease have only recently begun.
Questions/purposes
The purpose of our review was to assess the use of molecular biomarkers in the pathophysiology of hip OA, including (1) diagnosis; (2) disease staging; and (3) prognosis. We additionally aimed to summarize the available literature investigating the use of biomarkers in (4) prearthritic hip disease, including FAI and hip dysplasia.
Methods
We conducted a systematic review of molecular biomarkers associated with hip OA or prearthritic hip disease by searching four major electronic databases for keywords “hip”, “osteoarthritis”, “biomarker”, and all synonyms. The search terms “femoroacetabular impingement” and “hip dysplasia” were also included. The biologic source of biomarkers was limited to serum, plasma, urine, and synovial fluid. The literature search yielded a total of 2740 results. Forty studies met all criteria and were included in our review. Studies were categorized regarding their relevance to (1) diagnosis; (2) disease staging; (3) prognosis; and/or (4) prearthritic hip disease.
Results
Biomarker studies were characterized as relevant to diagnosis (16 studies), disease staging (15 studies), prognosis (11 studies), and prearthritic hip disease (three studies). Sixteen different biomarkers demonstrated associations relevant to the diagnosis of hip OA, 16 biomarkers demonstrated similar associations for disease staging, and six for prognosis. Six biomarkers seemed to be the most promising, demonstrating associations with hip OA in multiple studies, including: urinary level of type II collagen telopeptide (n = 5 studies), serum cartilage oligomeric protein (n = 4 studies), and serum C-reactive protein (n = 4 studies). Only three studies investigated the role of biomarkers in prearthritic hip disease, including two in FAI and one in unspecified etiology of pain. There were no studies about biomarkers in hip dysplasia.
Conclusions
Molecular biomarkers are increasingly investigated for their use in evaluating the pathophysiology of hip OA, but less so for prearthritic hip disease. Several biomarkers have demonstrated significant associations with hip OA across multiple studies. Further validation of these biomarkers is needed to assess their clinical use and potential application to prearthritic hip disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-015-4148-6) contains supplementary material, which is available to authorized users.
Introduction
Osteoarthritis (OA) is one of the most common conditions in medicine, affecting almost 27 million people in the United States alone. In particular, radiographic hip OA is present in approximately one in four individuals older than age 45 years [31]. The diagnosis of hip OA is made using radiographs and is based on features like narrowing of the femoroacetabular joint space, presence of osteophytes, and subchondral cysts. Hip OA was once thought to be predominantly primary in etiology; however, structural deformities increasingly are recognized as a potential risk factor for hip OA [26, 32, 33, 41]. Bony morphology associated with developmental dysplasia of the hip and femoroacetabular impingement (FAI) has been shown to be present in the majority of patients younger than age 50 years who present with hip OA [10]. The pathophysiology of hip OA is increasingly viewed as a continuum, progressing from an asymptomatic molecular phase, to a preradiographic, then radiographic phase, and finally, to end-stage OA [28]. Whereas hip OA may represent the end-stage clinical condition, the pathophysiology of hip OA appears to result from several distinct processes. Research efforts into prearthritic hip disorders offer the greatest potential to prevent or delay the onset of OA.
Molecular biomarkers of OA as intrinsic “indicators of pathologic processes” [3] have shown promise as a link between clinical status and disease pathology, yet no single OA biomarker has yet been shown to possess adequate sensitivity and specificity to allow for clinical use. Ongoing research attempts to define a group of molecular biomarkers with the greatest potential for future validation and use in clinical practice. Clinical research in the last decade has improved our understanding of and ability to treat patients with prearthritic hip disease, including FAI and hip dysplasia. Early detection of disease progression remains a challenge in these disorders and has generally relied on imaging methods as the gold standard for identification of early articular cartilage disease in the hip. However, despite improvements in radiographic and MR imaging, early identification of these patients remains challenging. Additionally, patients with prearthritic hip disease often have important and irreversible articular cartilage damage at the time of presentation, although they often have not had symptoms for long periods of time when they first present for evaluation.
The Osteoarthritis Research Society International committee has worked to standardize biomarker investigations by advocating use of the Burden of disease, Investigative, Prognosis, Efficacy of Intervention, Diagnosis (BIPED) criteria [45]—an acronym that represents categories in which biomarkers can be applied to clinical practice. Among the categories, three—diagnosis, burden of disease, and prognosis—are particularly relevant to improving the clinical management of patients with OA. A diagnostic marker reflects the early alterations in the joint that distinguish individuals with disease from those without disease, potentially allowing for early intervention. Markers of disease burden (subsequently referred to as disease staging) reflect OA severity, and prognostic markers correlate with the development and progression of disease over time. Each of these stages offers a valuable clinical opportunity, where identified biomarkers can serve as objective measures for diagnosis, disease staging, and predicting prognosis. However, the current literature on biomarkers in the pathophysiology of hip OA is large and spans several specialties making it difficult for the clinician to fully understand this topic.
The purpose of our systematic review therefore was to assess the use of molecular biomarkers in the pathophysiology of hip OA, including (1) diagnosis; (2) disease staging; and (3) prognosis. We additionally aimed to summarize the available literature investigating the use of biomarkers in (4) prearthritic hip disease, including FAI and hip dysplasia.
Materials and Methods
Literature Search
A literature search was conducted in four major electronic databases: MEDLINE (1966 to August 2014), Embase (1984 to August 2014), SCOPUS (1966 to August 2014), and CINAHL (1937 to August 2014) in August 2014. The search strategy, developed with the guidance of a clinical resource librarian, featured keywords “hip”, “osteoarthritis”, and “biomarker” in addition to all synonyms (eg, “biological marker”, “molecular marker”) and plural forms. Keywords “femoroacetabular impingement” and “hip dysplasia” were specifically included because of the importance of these prearthritic clinical states. The search also included the terms “serum”, “plasma”, “urine”, and “synovial fluid” because they represent sources of biomarkers with the greatest potential for clinical application. A detailed explanation of our search strategy is provided in the supplementary material (Appendix 1; supplemental materials are available with the online version of CORR®.).
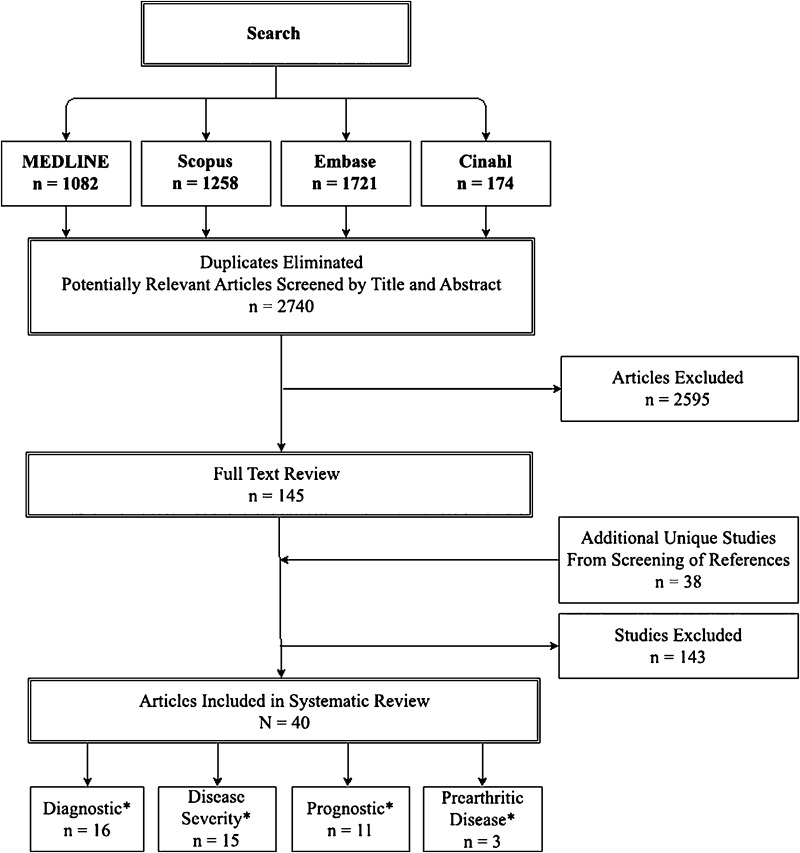
The computerized database search yielded 1082 MEDLINE results, 1721 Embase results, 1258 SCOPUS results, and 174 CINAHL results, for a total of 2740 titles (after duplicates were eliminated). Inclusion and exclusion criteria for the systematic review were established (Table 1), including data on biomarker levels in hip OA (or prearthritic hip disease) with comparison to control patients or varying severity of hip OA. Two reviewers (KMT, TWA) independently evaluated all search results by title and abstract. Studies identified for potential inclusion by either reviewer were then compiled and underwent full-text review for assessment of inclusion. Disagreements were resolved by a third reviewer (JJN), who made the final determination of inclusion or exclusion. Studies that presented data for OA of multiple joints were included only if hip subgroup data were available. Additionally, the bibliographies of relevant articles were crossreferenced but yielded no additional studies for final inclusion (Fig. 1).
Table 1.
Inclusion and exclusion criteria for systematic review
| Inclusion criteria | |
| 1. | Article studied OA and/or prearthritic conditions (eg, FAI, hip dysplasia) |
| 2. | Study evaluated ≥ 1 molecular biomarker |
| 3. | Biomarker levels were measured in serum, plasma, urine, or synovial fluid samples |
| 4. | Study compared patients with hip OA with either healthy control subjects, or, associated biomarker levels with radiographic phenotypes of hip OA (KL) grade, JSN, rapidly destructive OA, slowly progressive OA, atrophic OA, hypertrophic OA, et al. |
| 5. | Article presented original data |
| 6. | Hip-specific data presented |
| 7. | Language of publication = English |
| 8. | Human research, in vivo study |
| 9. | Age of subjects involved in research were adolescent or older |
| Exclusion criteria | |
| 1. | Arthropathies secondary to rheumatoid arthritis, spondyloarthropathy, posttraumatic arthritis, or crystalline arthropathies |
| 2. | Study evaluated effect of arthroplasty on biomarker levels |
| 3. | Study evaluated therapeutic interventions (eg, NSAIDs, hyaluronic acid, intraarticular corticosteroids) on biomarker levels |
| 4. | Study focused on imaging markers, genetic markers, or haplotype analysis |
| 5. | Study compared patients with OA to patients with other hip pathology (eg, femoral neck fracture, osteonecrosis, multisite OA) and/or did not include a healthy control group |
| 6. | Abstract-only publications |
OA = osteoarthritis; FAI = femoroacetabular impingement; KL = Kellgren-Lawrence; JSN = joint space narrowing; NSAIDs = nonsteroidal antiinflammatory drugs.
Fig. 1.
Flowchart shows literature search and screening strategy (inclusion/exclusion) of articles for study consideration. *Number includes studies that overlapped between categories.
Forty studies met final inclusion and exclusion criteria and are summarized in supplementary material (Appendix 2; supplemental materials are available with the online version of CORR®.). Relevant data were extracted by two reviewers (KMT, TWA), including parameters of the study population, study design, biomarker type, biomarker source, assay methods, and statistical analyses of biomarker concentrations in different patient groups. Articles were categorized according to their clinical relevance to hip OA as diagnosis (n = 16), disease staging (n = 15), prognosis (n = 11), or prearthritic hip disease (n = 3) (40 total studies, five studies in more than one category). We summarized the literature relative to the four categories used in our review and included any biomarker with a significant association (generally p < 0.05) in definitive analysis of given study in any of the four categories (Tables 2–5); biomarkers failing to show statistically significant associations in any category are summarized in supplementary materials (Appendices 3–5, supplemental materials are available with the online version of CORR ®.).
Table 2.
Biomarkers for the diagnosis of hip OA
| Marker | Source | Study | Definition of hip OA | Hip OA cohort, number (mean age ± SD [years]; men:women) | Control cohort, number (mean age ± SD [years]; men:women) | Hip OA biomarker levels, mean (± SD) ng/mL (unless otherwise specified) | Control biomarker levels, mean (± SD) ng/mL (unless otherwise specified) | Statistical association | Effect size (Glass’ delta) |
|---|---|---|---|---|---|---|---|---|---|
| CTX-II | Urine | Garnero et al., 2003 [19]* | ACR criteria | 40 (64 ± 12; 17:23) | 65 (63 ± 8; 27:38) | 492 (± 232) ng/mmol Cr | 342 (± 141) ng/mmol Cr | Yes; OA > controls; p < 0.001 | 2.2 |
| Jung et al., 2004 [22] | XR and clinical criteria NS | 51 (61 ± 10; 31:21) | 48 (66 ± 8; 19:29) | 597 (± 499) ng/mmol | 190 (± 109) ng/mmol | Yes; OA > controls; p < 0 .001 | 3.7 | ||
| Reijman et al., 2004 [37]* | K-L grade 2–4 | 123 (70 ± 7; 51:72) | 1112 (66 ± 7; 466:646) | 231.5 (NS) ng/mmol Cr | 172.0 (NS) ng/mmol Cr | Yes; OA > controls; p < 0.001 | N/A | ||
| CRP | Serum | Conrozier et al., 2000 [11] | ACR criteria | 45 (65 ± NS; 21:24) | 33 (42 ± NS; NS) | 2930 (± 3030) | 1400 (± 1610) | Yes; OA > controls; p = 0.006 | 1.0 |
| Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 2200 (± 1600) | 2800 (± 2800)° | No; NS | −0.2 | ||
| Hulejová et al., 2007 [21] | THA for OA K-L grade 3–4 | 55 (68 ± 10; 9:46) | 30 (56 ± 11; 8:22) | 9800 (± 13000) | 1200 (± 300) | Yes; OA > controls; p < 0.001 | 28.7 | ||
| TGF-β1 | Serum | Nelson et al., 2009 [34] | K-L grade 2–4; | 68 (NS; NS) | 241 (NS; NS) | 16.4 (NS) | 16.5 (NS) | No; p = 0.713 | N/A |
| JSN NS | 80 (NS; NS) | 226 (NS; NS) | 2.8 (NS) Ln ng/mL | 2.8 (NS) Ln ng/mL | No; p = 0.857 | N/A | |||
| Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 16.7 (13.1–21.3)† | 8.6 (6.3–11.7)† | Yes; OA > controls; p = 0.014 | N/A | ||
| Helix-II | Urine | Garnero et al., 2006 [18]* | ACR criteria | 40 (64 ± 12; 17:23) | 75 (63 ± 11; 28:47) | 281 (± 131) ng/mmol Cr | 200 (± 113) ng/mmol Cr | Yes; OA > controls; p = 0.004 | 0.7 |
| DPD | Urine | Stewart et al., 1999 [43] | Hip pain; radiographic OA NS | 28 (69 ± 6; 0:30) | 26 (70 ± 6; NS) | 24.3 (± 12.7) nmol/mmol Cr | 17.3 (± 15.3) nmol/mmol Cr | Yes; OA > OP and controls; p = 0.001; Yes; PYD:DPD ratio | 0.5 |
| Garnero et al., 2003 [19]* | ACR criteria | 40 (64 ± 12; 17:23) | 65 (63 ± 8; 27:38) | 3.2 (± 1.3) nmol/mmol Cr | 3.5 (± 0.9) nmol/mmol Cr | No; p = 0.3 | −0.3 | ||
| PYD | Urine | Stewart et al., 1999 [43] | Hip pain; radiographic OA NS | 30 (69 ± 6; 0:30) | 30 (70 ± 6; NS) | 90.8 (± 30.6) nmol/mmol Cr | 59.3 (± 15.1) nmol/mmol Cr | Yes; OA > OP and controls; p < 0.001; yes; PYD:DPD ratio | 2.1 |
| MMP-3 | Serum | Hulejová et al., 2007 [21] | THA for OA K-L grade 3–4 | 55 (68 ± 10; 9:46) | 30 (56 ± 11; 8:22) | 91.6 (± 20.3) pg/mL | 26.4 (± 10.5) pg/mL | Yes; OA > Controls; p = 0.03 |
6.2 |
| YKL-40 | Serum | Conrozier et al., 2000 [11] | ACR criteria | 45 (65 ± NS; 21:24) | 33 (42 ± NS; NS) | 90.3 (± 8.2) | 66.9 (± 8.2) | Yes; OA > controls; p = 0.035 |
2.0 |
| AAT | Serum | Olszewska-Slonina et al., 2013 [36] | Clinical diagnosis, K-L grade 3–4 | 46 (NS; 15:31) | 54 (63 ± 14; 26:28) | 0.9 (± 0.3) mg inhibited trypsin/mL blood serum | 0.7 (± 0.4) mg inhibited trypsin/mL blood serum | Yes; OA > control; p = 0.003 | 0.5 |
| CAT-D | Serum | Olszewska-Slonina et al., 2013 [36] | Clinical history, K-L grade 3–4 | 46 (NS; 15:31) | 54 (63 ± 14; 26:28) | 11.1 (± 4.8) nM released tyrosine/mg protein/min |
23.7 (± 13.1) nM released tyrosine/mg protein/min |
Yes; OA < controls; p < 0.001 | −1.0 |
| IL-10 | Serum | Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 2.2 (1.0–4.8)† pg/mL | 4.6 (3.3–6.3)† pg/mL | Yes; OA < controls; p = 0.029 | N/A |
| IL-12p70 | Serum | Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 0.6 (0.4–1.1)† pg/mL | 2.7 (1.5–5.0)† pg/mL | Yes; OA < controls; p = 0.007 | N/A |
| KS | Serum | Sweet et al., 1988 [44] | JSN, subchondral sclerosis, osteophytes | 31 (71 ± 4; 8:23) | 41 (62 ± NS; 22:19) | 475 (± 178) | 261 (± 51) | Yes; OA > controls; p < 0.001 | 4.2 |
| NO | Serum | Salvatierra et al., 1999 [38] | ACR criteria | 27 (60 ± 5; 10:17) | 12 (51 ± 7; 3:9) | 2.6 (± 0.5) μmol/L | 1.4 (± 0.6) μmol/L | Yes; ambulatory OA > control; p < 0.0001; yes; surgical > control; p < 0.0001 | 2.0 |
| MMP-9 | Serum | Hulejová et al., 2007 [21] | THA for OA K-L grade 3–4 | 55 (68 ± 10; 9:46) | 30 (56 ± 11; 8:22) | 259.8 (± 33.5) pg/mL | 68.2 (± 5.3) pg/mL | Yes; OA > controls; p < 0.001 | 36.2 |
| sIL-4R | Serum | Silvestri et al., 2006 [40] | K-L grade 3–4 | 34 (68 ± 10; 15:19) | 38 (67 ± 10; 8:30) | Median 49 (NS) pg/mL | 25.6 (NS) pg/mL | Yes; OA > controls; p < 0.001 | N/A |
| TIMP-1 | Serum | Chevalier et al., 2001 [9]* | K-L grade 2–3; ACR criteria | 29 (59 ± 11; 13:16) | 225 (40 ± 12; 109:116) | 529 (± 197.9) | 617 (± 107) | No; NS | −0.8 |
| Hulejová et al., 2007 [21] | THA for OA K-L grade 3–4 | 55 (68 ± 10; 9:46) | 30 (56 ± 11; 8:22) | 1.11 (0.06) | 1.14 (0.06) | No; NS | −0.5 | ||
| IL-6 | Serum | Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 4.4 (2.8–6.9)† pg/mL | 3.6 (2.1–6.1)† pg/mL | No; p = 0.6 | N/A |
| IL-1β | Serum | Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 0.4 (0.1–1.0)† pg/mL | 0.5 (0.3–0.9)† pg/mL | No; p = 0.6 | N/A |
| IL-8 | Serum | Koorts et al., 2012 [27] | Clinical diagnosis | 7 (NS; NS) | 19 (NS; NS) | 16.4 (11.4–23.5)† pg/mL | 14.2 (8.8–22.9)† pg/mL | No; p = 0.7 | N/A |
* Studies repeated in other categories; †geometric mean; AAT = alpha-1 antitrypsin; ACR = American College of Rheumatology; CAT-D = cathepsin D; Cr = creatinine; CRP = C-reactive protein; CTX-II = type II collagen telopeptide; DPD = deoxypyridinoline; Helix-II = type II collagen helical telopeptide; IL = interleukin; JSN = joint space narrowing; K-L = Kellgren-Lawrence; KS = keratan sulfate; Ln = natural log; MMP = matrix metalloproteinase; N/A = not applicable; NO = nitrous oxide; NS = not specified; OA = osteoarthritis; PYD = pyridinoline; sIL-4R = soluble IL-4 receptor; TGF-β1 = transforming growth factor β1; TIMP-1 = TIMP metalloproteinase inhibitor 1; XR = radiograph; YKL-40 = human cartilage glycoprotein 39.
Table 5.
Biomarkers for evaluation of prearthritic disorders
| Marker | Source | Study | Study group/control group | Definition of study group | Number | Age (mean years; ± SD) | Men:women ratio | Biomarker levels (mean; ± SD) | Statistical association | Effect size (Glass’ delta) |
|---|---|---|---|---|---|---|---|---|---|---|
| COMP | Serum | Bedi et al., 2013 [4] | FAI | Cam, pincer, or mixed FAI | 10 | 23.1 (6.4) | 10:0 | 240 (50) ng/mL | Yes; FAI > no FAI; p = 0.04 | 0.9 |
| Control (no FAI) | 19 | 22.3 (3.4) | 19:0 | 195 (50) ng/mL | ||||||
| Dragomir et al., 2002 [16] | Symptomatic/no OA | K-L grade OA = 0; Symptoms = groin pain; pain, aching or stiffness on most days | 54 | 60.2 (9.8) | 77:78 | Median 1104 (IQR 863–1415) ng/mL | Yes; general linear model of (Ln COMP); p = 0.046 | N/A | ||
| Control (asymptomatic/no OA) | 89 | Median 919 (IQR 771–1108) ng/mL | ||||||||
| Clinical signs/no OA | K-L grade OA = 0; Clinical signs = hip pain with flexion/internal rotation | 35 | 60.2 (9.8) | 77:78 | Median 1270 (IQR 870–1506) ng/mL | Yes; general linear model of (Ln COMP); p = 0.018 | N/A | |||
| Control (No clinical signs/no OA) | 105 | Median 924 (IQR 793–1131) ng/mL | ||||||||
| CRP | Serum | Bedi et al., 2013 [4] | FAI | Cam, pincer, or mixed FAI | 10 | 23.1 (6.4) | 10:0 | 3.1 (2.5) mg/L | Yes; FAI > no FAI; p < 0.001 | 3.1 |
| Control (no FAI) | 19 | 22.3 (3.4) | 19:0 | 0.9 (0.7) mg/L | ||||||
| FAC | Synovial fluid | Abrams et al., 2014 [2] | FAI | Tönnis OA ≥ 2 | 17 | 38.3 (11.1) | 5:12 | 1.2 (1.5) μg/mL | Yes; FAI > OA; p < 0.001 | 0.7 |
| Control (OA) | 17 | 59.2 (11.9) | 9:8 | 0.08 (1.6) μg/mL |
COMP = cartilage oligomeric protein; CRP = C-reactive protein; FAC = fibronectin-aggrecan complex; FAI = femoroacetabular impingement; IQR = interquartile range; K-L = Kellgren-Lawrence; Ln = natural log; N/A = not applicable; OA = osteoarthritis.
Sixteen “diagnosis” studies [9, 11, 18, 19, 21, 22, 25, 27, 34, 36–38, 40, 43, 44, 46] investigated the differences in the levels of 34 different biomarkers between patients with hip OA and control subjects without OA. Control groups were generally comprised of asymptomatic individuals without evidence of radiographic hip OA. Fifteen “disease staging” studies [1, 5, 6, 12–15, 18–20, 23, 42, 47–49] investigated the association between 41 different biomarker levels and severity of hip OA. Studies reported various methods to define hip OA severity, including rapidly destructive versus slowly progressive in six studies [1, 5, 12, 18, 19, 49]; atrophic versus hypertrophic in two studies [13, 14]; and radiographic OA classification in five studies [6, 23, 42, 47, 48]. Two studies [15, 20] analyzed minimum joint space width as a continuous variable relative to biomarker levels. Eleven “progression” studies [7–9, 15, 17, 24, 29, 30, 35, 37, 39] investigated the association of 13 different biomarker levels with the progression of hip OA. Of the 11 studies, four looked at progression of known hip OA [9, 15, 37, 39]; three studies investigated the progression to incident hip OA from unspecified prearthritic hip disease [8, 17, 29]; and four studies investigated both OA progression and incidence [7, 24, 30, 35]. Duration of followup to evaluate OA progression ranged from 1 year to 15 years.
Three “prearthritic hip disease” studies [2, 4, 16] investigated the role of three unique biomarkers in this population.
No statistical analysis or meta-analysis was performed given the heterogeneity of studies included. Glass’s delta (d) was calculated to quantify the effect size with values greater than 0.8 generally indicating large effects.
Results
A total of 33 different biomarkers demonstrated associations with hip OA.
Diagnosis
A total of 16 different biomarkers demonstrated differences between patients with hip OA and non-OA control subjects (Table 2) Although most biomarkers were assessed in only one “diagnosis” study, urinary level of type II collagen telopeptide (uCTX-II) [19, 22, 37] and serum C-reactive protein (CRP) [11, 21, 27] levels both were increased in patients with hip OA in more than one study. In three studies [19, 22, 37], uCTX-II levels were elevated in patients with hip OA 1.3 to 3.1 times that of the control group with associated effect size (d) of 2.2 to 3.7. Serum CRP level was elevated in patients with hip OA compared with control subjects in two studies (2.1–8.2 times greater than control levels, d 1.0–28.7) [11, 21] while failing to demonstrate an effect in another study [27]. Thirteen other biomarkers demonstrated differences in levels between OA and control patients in a single study (with approximately 70% demonstrating large effect sizes). Four biomarkers demonstrating associations with hip OA in other categories (disease staging, prognosis) failed to demonstrate associations for diagnosis of hip OA.
Disease Staging
Associations between hip OA severity and biomarker level were noted for 16 biomarkers (Table 3). Urinary CTX-II and serum CRP levels were the only biomarkers with associations demonstrated in more than one study. Elevations in biomarker levels were noted with increasing severity of disease for uCTX-II in two studies [18, 20] with an effect size (d) of 0.8 in one study [18]. Serum CRP levels were greater with more severe hip OA (1.3–2.9 times greater, effect size d = 1.2–1.8) than those with less severe OA. Increased levels of cartilage oligomeric protein (COMP) were associated with increasing hip OA severity in one study (effect size d = 4.9) [15] but not in three other studies [6, 14, 20]. However, a recent study [6] demonstrated increased levels of deamidated COMP (DCOMP) to be associated with increasing hip OA severity as measured by the Kellgren-Lawrence grade, whereas COMP demonstrated no association.
Table 3.
Biomarkers for disease staging of hip OA
| Marker | Source | Study | OA subgroup (or variable) and definition | Number | Age (mean years; ± SD) | Men:women ratio | Biomarker level (mean ng/mL; ± SD) | Statistical association | Effect size (Glass’ delta) |
|---|---|---|---|---|---|---|---|---|---|
| CRP | Serum | Garnero et al., 2005 [20] | JSW (continuous) – mean (± SD) = 2.3 (0.8) mm | 376 | 62.4 (7) | 152:224 | 3070 (4580) | No | 1.3 |
| Berger et al., 2005 [5] | RDOA (NS) | 18 | 72.8 (9.6) | 2:16 | 14.3 (9.6) | Yes; RDOA > SPOA; p = 0.01 | 1.9 | ||
| SPOA (NS) | 20 | 62.8 (11.1) | 4:16 | 6.8 (6.1) | |||||
| Conrozier et al., 1998 [12] | RDOA = JSN > 1 mm/year | 10 | 71.2 (10.6) | 4:6 | 5610 (4750) | Yes; RDOA > SPOA; p = 0.01 | 1.5 | ||
| SPOA = JSN < 0.2 mm/year | 23 | 60.5 (11.8) | 9:14 | 1940 (1980) | |||||
| Conrozier et al., 2004 [14] | Atrophic OA = lack of osteophytes | 25 | 67.0 (2.0) | 4:21 | 4400 (800) | No | N/A | ||
| Hypertrophic = presence of osteophytes | 25 | 62.4 (1.8) | 12:13 | 3500 (600) | |||||
| CTX-II | Urine | Garnero et al., 2006 [18]* | RDOA = JSN > 1 mm/year | 12 | 70 (8) | 5:7 | 612 (218) ng/mmol Cr | Yes; RDOA > SPOA; p = 0.015 | 0.8 |
| SPOA= JSN < 0.2 mm/year | 28 | 63 (9) | 17:23 | 441 (221) ng/mmol Cr | |||||
| Garnero et al., 2005 [20] | JSW (continuous) – mean (± SD) = 2.27 (± 0.80) mm | 376 | 62.4 (7) | 152:224 | 317 (211) ng/mmol Cr | Yes; B = –0.001†; p = 0.0009 | N/A | ||
| COMP | Serum | Garnero et al., 2005 [20] | JSW (continuous variable) mean (± SD) = 2.3 (± 0.8) mm | 376 | 62.4 (7) | 152:224 | 10.77 (2.7) Units/L | No | N/A |
| Conrozier et al., 2004 [14] | Atrophic OA = lack of osteophytes | 25 | 67.0 (2.0) | 4:21 | 570.4 (86.6) ng/mL | No | 4.9 | ||
| Hypertrophic = presence of osteophytes | 25 | 62.4 (1.8) | 12:13 | 383.7 (37.9) ng/mL | |||||
| Catterall et al., 2012 [6] | K-L grade 0–1 | 48 | NS | NS | 7.6 (0.5) Ln pg/mL | No; K-L0–1 versus K-L2/3/4 | N/A | ||
| K-L grade 2 | 200 | NS | NS | 7.63 (0.22) Ln pg/mL | No; K-L2 versus K-L3/4 | ||||
| K-L grade 3 | 99 | NS | NS | 7.67 (0.3) Ln pg/mL | No; K-L3 versus K-L4 | N/A | |||
| K-L grade 4 | 102 | NS | NS | 7.71 (0.25) Ln pg/mL | |||||
| Conrozier et al., 1998 [15]* | JSW (continuous – mean (± SD) = 1.80 (± 1.04) mm at baseline | 48 | 56.3 (14.1) | 23:25 | 7200 (1000) ng/mL | Yes; baseline; COMP correlated with baseline JSW (r = 0.4; p = 0.001) | N/A | ||
| JSW (continuous) – mean (± SD) = 2.32 (NS) mm at 1 year | 48 | 56.3 (14.1) | 23:25 | 7600 (14) ng/mL | Yes; COMP at 1 year correlated with 1 year JSW (r = 0.4; p = 0.02) | ||||
| DCOMP | Serum | Catterall et al., 2012 [6] | K-L grade 0–1 | 48 | NS | NS | 1.9 (1.1) Ln pg/mL | N/A | |
| K-L grade 2 | 200 | NS | NS | 2.3 (0.8) Ln pg/mL | Yes; K-L0–1 < K-L2; p < 0.01 | ||||
| K-L grade 3 | 99 | NS | NS | 2.3 (0.7) Ln pg/mL | Yes; K-L0–1 < K-L3; p < 0.001 | ||||
| K-L grade 4 | 102 | NS | NS | 2.4 (0.7) Ln pg/mL | Yes; K-L0–1 < K-L4; p < 0.001 | ||||
| Helix-II | Urine | Garnero et al., 2006 [18]* | RDOA = JSN > 1 mm/year | 12 | 70 (8) | 5:7 | 396 (160) ng/mmol Cr | Yes; RDOA > SPOA; p = 0.002 | 1.4 |
| SPOA = JSN < 0.2 mm/year | 28 | 63 (9) | 17:23 | 232 (118) ng/mmol Cr | |||||
| IL-6 | Serum | Abe et al., 2014 [1] | RDOA (NS) | 33 | 75 (6) | 6:28 | 783 (25–6282) median (range) pg/mL | Yes; RDOA > SPOA; p < 0.01 | N/A |
| SPOA (NS) K-L0–1 | 20 | NS | NS | 44 (0–1127) median (range) pg/mL | Yes; K-L3–4 > K-L0–1; p < 0.0001 | ||||
| SPOA (NS) K-L3–4 | 37 | NS | NS | 524 (48–3427) median (range) pg/mL | |||||
| Stannus et al., 2010 [42] | Altman Grade JSN/osteophytes Grade 0 | 102 | NS | NS | NS | NS | N/A | ||
| Grade 1 | 91 | NS | NS | NS | NS | ||||
| Grade ≥ 2 | NS | NS | |||||||
| IL-1β | Serum | Abe et al., 2014 [1] | RDOA (NS) | 33 | 75 (6) | 6:28 | 261 (33–6223) median (range) pg/mL | Yes; RDOA > SPOA; p < 0.001 | N/A |
| SPOA (NS) K-L0–1 | 20 | NS | NS | 50 (0–413) median (range) pg/mL | Yes; K-L3–4 > K-L0–1; p = 0.004 | ||||
| SPOA (NS) K-L3–4 | 37 | NS | NS | 86 (8–5675) median (range) pg/mL | |||||
| IL-8 | Serum | Abe et al., 2014 [1] | RDOA (NS) | 33 | 75 (± 6) | 6:28 | 3305 (330–9534) median (range) pg/mL | Yes; RDOA > SPOA; p < 0.001 | N/A |
| SPOA (NS) K-L0–1 | 20 | NS | NS | 163 (42–737) median (range) pg/mL | Yes; K-L3–4 > K-L0–1; p < 0.001 | ||||
| SPOA (NS) K-L3–4 | 37 | NS | NS | 190 (42–5020) median (range) pg/mL | |||||
| BSP | Serum | Conrozier et al., 1998 [15]* | JSW (continuous) – mean (± SD) = 1.8 (1.0) | 48 | 56.3 (14.1) | 23:25 | 7700 (1000) | No; not correlated with JSW; NS | |
| Atrophic OA = lack of osteophytes | NS | NS | NS | 167.4 (14.5) | Yes; atrophic > hypertrophic; p = 0.006 | 1.2 | |||
| Hypertrophic = presence of osteophytes | NS | NS | NS | 139 (23.5) | |||||
| C6S/C4S ratio | Synovial fluid |
Yamada et al., 1999 [47] | Pre-OA–JOA classification | 0 | N/A | N/A | N/A | N/A | |
| Early OA–JOA classification | 17 | 36.9 (13.2) | 0:17 | 3.4 (0.8) | Yes; ratio C6S/C4S terminal < early; p < 0.01 Yes; ratio C6S/C4S advanced < early; p < 0.05 | ||||
| Advanced OA–JOA classification | 6 | 52.2 (12) | 1:5 | 2.4 (0.2) | −1.3 | ||||
| Terminal OA–JOA classification | 27 | 62.4 (11.2) | 2:25 | 1.9 (0.4) | −1.9 | ||||
| Primary OA | 8 | NS | NS | NS | No; primary versus secondary OA | ||||
| Secondary OA (primary DDH) | 42 | NS | NS | NS | |||||
| CPII | Serum | Conrozier et al., 2007 [13] | Atrophic OA–lack of osteophytes | 14 | NS | NS | 93.7 (50.7) | Yes; p = 0.004§ | −0.5 |
| Hypertrophic–presence of osteophytes | 42 | NS | NS | 132.6 (75.2) | |||||
| ICTP | Serum | Berger et al., 2005 [5] | RDOA (NS) | 18 | 72.8 (9.6) | 2:16 | 13.2 (5.6) | Yes; RDOA > SPOA; p = 0.002 | 6.9 |
| SPOA (NS) | 20 | 62.8 (11.1) | 4:16 | 3.6 (1.4) | |||||
| L2-KS | Synovial fluid | Yamada et al., 2000 [48] | Early OA–JOA classification | 6 | 26.3 (10.9) | 1:5 | 17.7 (8.86) nmoles/mL | Yes; early versus advanced, p < 0.001; early versus terminal, p < 0.001 | |
| Advanced OA–JOA classification | 12 | 44.2 (7.9) | 1:11 | 10.8 (6.97) nmoles/mL | −0.8 | ||||
| Terminal OA–JOA classification | 32 | 62.7 (10.9) | 2:30 | 7.25 (3.92) nmoles/mL | −1.2 | ||||
| Leptin | Serum | Stannus et al., 2010 [42] | Altman Grade–JSN/osteophytes Grade 0 | 102 | NS | NS | 7.5 μg/mL† | Yes; p < 0.001 | N/A |
| Grade 1 | 91 | NS | NS | 12 μg/mL† | |||||
| Grade ≥ 2 | 21 μg/mL† | ||||||||
| TNFα | Serum | Abe et al., 2014 [1] | RDOA (NS) | 33 | 75 (6) | 6:28 | 348 (27–7715) median (range) pg/mL | Yes; RDOA > SPOA; p < 0.001 | N/A |
| SPOA (NS) K-L 0–1 | 20 | NS | NS | 90 (3–500) median (range) pg/mL | Yes; K-L3–4 > K-L0–1; p = 0.002 | ||||
| SPOA (NS) K-L 3–4 | 37 | NS | NS | 207 (63–5290) median (range) pg/mL | |||||
| TRACP-5b | Synovial fluid | Yamaguchi et al., 2014 [49] | RDA = end-stage OA–JOA classification | 21 | 70 (NS) | 2:19 | 1540 (NS) mU/dL‡ | Yes; RDA > DDH; p < 0.0001; Yes; RDA > advanced OA; p = 0.0007 | N/A |
| DDH (any OA–JOA classification) | 20 | 56 (NS) | 2:18 | 340 (NS) mU/dL‡ | |||||
| DDH (early OA) = JSW ≥ 2 mm throughout weightbearing area | 7 | 56 (NS) | 2:18 | 260 (NS) mU/dL‡ | Yes; early OA < advanced OA; p = 0.029 | ||||
| DDH (advanced OA) = JSW < 2 mm or complete loss of JS | 13 | 440 (NS) mU/dL‡ | |||||||
| TIMP-1 | Serum | Conrozier et al., 2004 [14] | Atrophic OA = lack of osteophytes | 25 | 67.0 (2.0) | 4:21 | 745.3 (27.6) | No; atrophic versus hypertrophic | 1.0 |
| Hypertrophic = presence of osteophytes | 25 | 62.4 (1.8) | 12:13 | 700.6 (46.5) | |||||
| DPD | Urine | Garnero et al., 2003 [19]* | RDOA = JSN > 1 mm/year | 12 | 70 (8) | 5:7 | RDOA = 3.3 (1.0) nmol/mmol Cr | No; RDOA versus SPOA; NS | 0.1 |
| SPOA = JSN < 0.2 mm/year | 28 | 63 (9) | 17:23 | SPOA = 3.2 (1.4) nmol/mmol Cr | |||||
| MMP3 | Serum | Garnero et al., 2005 [20] | JSW (continuous variable) mean (± SD) = 2.27 (0.80) mm | 376 | 62.4 (7) | 152:224 | 25.4 (28.6) | No; not correlated with JSW | N/A |
| YKL-40 | Serum | Garnero et al., 2005 [20] | JSW (continuous variable) mean (± SD) = 2.27 (0.80) mm | 376 | 62.4 (7) | 152:224 | 87.9 (85.7) | No; not correlated with JSW | N/A |
* Studies repeated in other categories; †estimated value; ‡median reported; B = coefficient of independent variable in multivariate linear/logistic regression model; §continuous variable analyzed by linear regression; BSP = bone sialoprotein; C4S = chondroitin-4-sulfate; C6S = chondroitin-6-sulfate; COMP = cartilage oligomeric protein; CPII = C-propeptide; Cr = creatinine; CRP = C-reactive peptide; CTX-II = type II collagen telopeptide; DCOMP = deaminated cartilage oligomeric protein; DDH = developmental dysplasia of the hip; DPD = deoxypyridinoline; Helix-II = type II collagen helical telopeptide; ICTP = crosslinking C-terminal telopeptide; IL = interleukin; JOA = Japanese Orthopedic Association; JS = joint space; JSN = joint space narrowing; JSW = joint space width; K-L = Kellgren-Lawrence; L2-KS = B-galactosyl-(1-4)-6-0-sulfo-N-acetylglucosamine (L2 fragment of keratan sulfate); Ln = natural log; MMP = matrix metalloproteinase; N/A = not applicable; NS = not specified; OA = osteoarthritis; RDA = rapidly destructive arthrosis; RDOA = rapidly progressive OA; SPOA = slowly progressive OA; TIMP-1 = TIMP metalloproteinase inhibitor 1; TNFα = tumor necrosis factor-alpha; TRACP-5b = tartrate-resistant acid phosphatase 5b; YKL-40 = human cartilage glycoprotein 39.
Prognosis
Six different biomarkers were associated with hip OA progression (Table 4), including elevations in COMP (three studies [7, 15, 24]) and decreases in 25-OH Vitamin D (two studies [8, 29]). However, all COMP studies demonstrated relatively small effect sizes (d < 0.3).
Table 4.
Biomarkers for evaluating prognosis of hip OA
| Marker | Source | Study | Definition of OA | Baseline group | Duration (years) | Imaging outcome | Groups | Number | Age (mean years; ± SD) | Men:women ratio | Biomarker baseline levels (mean ng/mL; ± SD) | Biomarker rnidpoint levels (mean ng/mL; ± SD) | Statistical association | Effect size (Glass’ delta) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMP | Serum | Chaganti et al., 2008 [7] | Radiographic criteria | No OA | XR at 8, BM at 6 | Incident OA | No OA | 169 | 69.6 (3.7) | 0:169 | 11.1 (3.3) U/L | 10.8 (3.0) U/L | No; no OA (% change) versus incident OA (% change) | −0.2 |
| Incident OA | 167 | 70.8 (4.6) | 0:167 | 10.6 (2.9) U/L | 12.3 (3.7) U/L | Yes; OR† = 1.7 (95% CI, 1.2–2.3) incident OA/1 SD increase | ||||||||
| OA | XR at 8, BM at 6 | Radiographic OA progression or THA | Stable OA | 173 | 71.8 (5.5) | 0:173 | 10.7 (3.2) U/L | 11.6 (3.5) U/L | No; stable OA (% change) versus progressive OA (% change) | 0.2 | ||||
| Progressive OA | 168 | 72.2 (5.5) | 0:168 | 11.2 (3.6) U/L | 11.1 (3.6) U/L | Yes; OR† = 0.7 (95% CI, 0.5–0.9) progressive OA/1 SD increase | ||||||||
| Conrozier et al., 1998 [15]* | ACR criteria | OA | XR at 1 | Interval JSN (continuous) mean mm/year = 0.5 | Progression | 48 | 56.4 (14.1) | 23:25 | 7.7 (1.0) ug/mL | 7.6 (1.4) μg/mL | Yes; baseline COMP correlated with interval JSN (r = 0.38, p = 0.002) | N/A | ||
| Kelman et al., 2006 [24] | Radiographic criteria, modified Croft | No OA | XR at 8 | Incident OA | No OA | 194 | NS (NS) | 0:194 | 11.4 (4.3) U/L | N/A | Yes; incident OA > No OA, p < 0.05 per 1 SD | 0.3 | ||
| Incident OA | 186 | NS (NS) | 0:186 | 12.8 (6.6) U/L | N/A | |||||||||
| OA | Radiographic OA progression or THA | Stable OA | 197 | NS (NS) | 0:197 | 11.4 (4.4) U/L | N/A | No; stable versus progressive OA | 0.1 | |||||
| Progressive OA | 194 | NS (NS) | 0:194 | 11.8 (5.2) U/L | N/A | |||||||||
| 25-OH Vit D | Serum | Chaganti et al., 2010 [8] | Radiographic criteria | No OA | XR at 8, BM at 6 |
Incident OA | No OA | 1104 | 77.2 (5.3) | 1104:0 | 26.0 (7.8) | N/A | Yes; OR† = 1.39 (95% CI, 1.11–1.74) increased prevalence of RHOA/ 1 SD decrease* | −0.3 |
| Incident OA | 23.4 (6.7) | N/A | ||||||||||||
| Lane et al., 1999 [29] | Radiographic criteria | No hip OA | XR at 8 | Incident OA | No OA | 138 | 71.2 (4.9) | 0:138 | 26.9 (8.0) | N/A | No; but yes, low/mid > high tertile for JSN, p = 0.03/0.04 | −0.2 | ||
| Incident OA | 35 | 69.7 (3.6) | 0:35 | 25.0 (9.6) | N/A | |||||||||
| NTX | Serum | Chaganti et al., 2008 [7] | Radiographic criteria | No OA | XR at 8, BM at 6 | Incident OA | No OA | 169 | 69.6 (3.7) | 0:169 | 20.2 (9.0)‡ | 19.5 (5.7)‡ | No; no OA (% change) versus incident OA (% change) | 0.0 |
| Incident OA | 167 | 70.8 (4.6) | 0:167 | 20.5 (8.0)‡ | 21.0 (7.9)‡ | NS | ||||||||
| OA | XR at 8, BM at 6 | OA progression (JSN ≥ 0.5 mm, increase OA grade) | Stable OA | 173 | 71.8 (5.5) | 0:173 | 20.7 (7.3)‡ | 20.4 (7.6)‡ | No; stable (% change) versus progressive OA (% change) | 0.0 | ||||
| Progressive OA | 168 | 72.2 (5.5) | 0:168 | 20.9 (7.2)‡ | 20.3 (6.5)‡ | NS | ||||||||
| Kelman et al., 2006 [24] | Radiographic criteria, modified Croft | No OA | XR at 8 | Incident OA | No OA | 199 | 69.7 (3.8) | 0:199 | 21.8 (8.6)‡ | N/A | Yes; incident OA > no OA, p < 0.05 per 1 SD | 0.2 | ||
| Incident OA | 198 | 70.9 (4.6) | 0:198 | 23.8 (12.3)‡ | N/A | |||||||||
| OA | Radiographic OA progression or THA | Stable OA | 199 | 71.9 (5.8) | 0:199 | 24.8 (13.3)‡ | N/A | No; stable versus progressive OA | 0.0 | |||||
| Progressive OA | 197 | 72.3 (5.4) | 0:197 | 24.9 (11.9)‡ | N/A | |||||||||
| CTX-II | Urine | Reijman et al., 2004 [37]* | K-L grade ≥ 2 | OA | XR at 7 | Progressive OA 1 (≥ 1 mm JSN) | Stable RHOA | 50 | NS (NS) | NS | NS (NS) | N/A | NS | N/A |
| Progressive RHOA | 73 | NS (NS) | NS | NS (NS) | N/A | Yes; OR† = 1.3 (95% CI, 1.0–1.8) progressive OA/1 SD unit increase | ||||||||
| TIMP-1 | Serum | Chevalier et al., 2001 [9]* | K-L grade 2–3 | OA | XR and BM at 1 | JSN (continuous)–mean = 0.4 mm | Progression | 29 | 59 (11) | 13:16 | 529.7 (197.9) | 493.5 (190.2) | Yes; baseline TIMP correlated with log-transformed mean JSN; R = 0.45, p = 0.02 | |
| No progression (< 0.6 mm) Progression (> 0.6 mm) | Nonprogressors | 10 | NS | NS | 589 (217.6) | 651.3 (285.3) | Yes; no progression > progression, p = 0.01 | −0.8 | ||||||
| Progressors | 19 | NS | NS | 416 (73.9) | 441.7 (83.2) | |||||||||
| VCAM-1 | Serum | Schett et al., 2009 [39] | THA for OA; OA diagnosis based on 2 successive XR | NS | XR at 15 | Progression to THA | No THA (no OA/progression) | 852 | NS (NS) | NS | NS (NS) | N/A | Yes; OR† = 1.7 (95% CI, 1.3–2.3) THA performed/1 SD unit increase, p < 0.001 | N/A |
| THA (OA progression) | 60 | NS (NS) | NS | NS (NS) | N/A | |||||||||
| CRP | Serum | Engström et al., 2009 [17] | First hip arthroplasty for OA | No OA | Average, 12 | Incident OA | No OA | 5044 | 57.5 (6.0) | 2100:2944 | 1.40 (NS) | N/A | No; no versus incident OA | N/A |
| Incident OA | 120 | 60 (5.1) | 40:80 | 1.64 (NS) | N/A | |||||||||
| TGF-β1 | Serum | Nelson et al., 2010 [35] | K-L grade ≥ 1 from K-L = 0 | No OA | XR at 6 | Incident OA 1 | No OA | 564 | NS (NS) | NS | NS (NS) | N/A | N/A | |
| Incident OA | 54 | NS (NS) | NS | NS (NS) | N/A | No; HR† = 1.1 (0.3–3.5) | ||||||||
| K-L grade ≥ 2 from K-L grade < 2 | No OA | Incident OA 2 | No OA | 573 | NS (NS) | NS | NS (NS) | N/A | ||||||
| Incident OA | 45 | NS (NS) | NS | NS (NS) | N/A | No; HR† = 0.6 (0.3–1.5) | ||||||||
| Increase by ≥1 K-L from K-L ≥1 | OA | Progressive OA 1 | Stable OA | 571 | NS (NS) | NS | NS (NS) | N/A | ||||||
| Progressive OA | 47 | NS (NS) | NS | NS (NS) | N/A | No; HR† = 1.0 (0.5–2.3) | ||||||||
| Increase by ≥ 1 K-L grade from K-L grade ≥ 2 | OA | XR at 6 | Progressive OA 2 | Stable OA | 608 | NS (NS) | NS | NS (NS) | N/A | |||||
| Progressive OA | 10 | NS (NS) | NS | NS (NS) | N/A | No; HR† = 2.7 (0.3–24.3) | ||||||||
| Progressive JSN | 7 | NS (NS) | NS | NS (NS) | N/A | No; HR† = 4.1 (0.6–27.7) | ||||||||
| BSP | Serum | Conrozier et al., 1998 [15]* | ACR criteria | OA | XR at 1 | Interval JSN–mean mm = 0.5 | Progression | 48 | 56.4 (14.1) | 23:25 | 143 (27.3) | 160 (36.4) | No; no correlation with interval JSN | N/A |
* Studies repeated in other categories; †adjusted multivariate regression model, see primary source for full details; ‡units= nM bone collagen equivalents (BCE); 25(OH) Vit D=25-hydroxy Vitamin D; ACR = American College of Rheumatology; BCE = bone collagen equivalents; BM = biomarker; BSP = bone sialoprotein; CI = confidence interval; COMP = cartilage oligomeric protein; CRP = C-reactive protein; CTX-II = type II collagen C telopeptide; HR = hazards ratio; JSN = joint space narrowing; K-L = Kellgren-Lawrence; N/A = not applicable; NS = not specified; NTX = N-terminal telopeptide; OA = osteoarthritis; OR = odds ratio; r = Pearson correlation coefficient; RHOA = radiographic hip osteoarthritis; TGF-β1 = transforming growth factor β1; TIMP = TIMP metalloproteinase inhibitor; VCAM-1 = vascular cell adhesion molecule 1; XR = radiograph.
Prearthritic Hip Disease
Bedi et al. [4] demonstrated elevations in COMP (effect size d = 0.9) and CRP levels (effect size d = 3.1) in athletes with symptomatic FAI compared with athletes with asymptomatic (radiographically normal) hips (Table 5). Dragomir et al. [16] found increased levels of COMP in a cohort of patients reporting recent hip pain or pain during hip examination (without a specific prearthritic diagnosis) compared with normal control subjects (insufficient detail to allow calculation of effect size). Abrams et al. [2] demonstrated lower fibronectin aggrecan complex in patients with FAI compared with a cohort of patients with hip OA (effect size d = 0.7). No studies investigated biomarkers in a prearthritic population, which included patients with hip dysplasia.
Discussion
In this systematic review, we found that although more than 70 biomarkers have been investigated in hip OA, none have been validated for clinical use. The current literature on biomarkers in the pathophysiology of hip OA is large and spans several specialties making it difficult for the clinician to fully understand this topic. Several biomarkers appear to be particularly promising, having demonstrated important associations with the pathophysiology of hip OA in more than a single study (Table 6). Biomarkers are generally viewed as a byproduct of the underlying disease process rather than a cause of the disease. Urinary CTX-II has demonstrated differences (five of five studies) including those relevant to “diagnosis”, “disease staging”, and “prognosis”. CTX-II is a major component of cartilage; fragments of CTX-II found in serum or urine are derived from turnover of cartilage and are thought to reflect the structural integrity of cartilage. Other promising biomarkers include serum COMP (in five of eight studies, and, additionally DCOMP in one study) and serum CRP (five of nine studies). CRP is an acute phase protein produced by the liver and acts as a nonspecific molecular marker of systemic inflammation. COMP is an extracellular matrix protein found abundantly in cartilage and released with cartilage breakdown. However, recent evidence suggests crossover of biomarkers between often used simplified pathways, including cartilage metabolism, bone metabolism, and inflammation. For example, several studies have demonstrated an associated COMP association with inflammatory biomarker clusters, suggesting COMP may not be specific to cartilage breakdown but also associated with inflammatory pathways [6]. Additionally, uHelix-II exhibited statistically associations with hip OA in both diagnosis and disease staging within a single study. Helix-II is a helical fragment derived from matrix metalloproteinase breakdown of type II collagen; urinary levels have been studied as a potential biomarker specific for type II collagen degradation and pathologies involving cartilage matrix turnover. This group of biomarkers offers great promise to investigations of prearthritic hip disease. Biomarkers have not been well investigated with regard to prearthritic hip conditions. Molecular biomarkers may allow for early diagnosis and monitoring for disease progression in patients with prearthritic hip conditions. Hip dysplasia and FAI are increasingly recognized and treated to improve patient function and potentially avoid progression of hip OA. The pathophysiology and associated relevant biomarkers may be different between FAI and hip dysplasia.
Table 6.
Summary of significant biomarkers by category
| Diagnosis (n = 16) |
Significant studies/total studies | Severity of hip OA (n = 16) |
Significant studies/total studies | Prognosis (n = 6) |
Significant studies/total studies | Prearthritic hip disease (n = 3) |
Significant studies/total studies |
|---|---|---|---|---|---|---|---|
| uCTX-II | 3/3 | sCRP | 2/4 | sCOMP | 3/3 | sCOMP | 2/2 |
| sCRP | 2/3 | uCTX-II | 2/2 | s25-OH Vit D | 2/2 | sCRP | 1/1 |
| sTGF-B1 | 1/2 | sCOMP | 1/4 | sNTX | 1/2 | sfFAC | 1/1 |
| uHelix-II | 1/1 | sDCOMP | 1/1 | uCTX-II | 1/1 | ||
| uDPD | 1/2 | uHelix-II | 1/1 | sTIMP-1 | 1/1 | ||
| uPYD | 1/1 | sIL-6 | 1/2 | sVCAM-1 | 1/1 | ||
| sMMP-3 | 1/1 | sIL-1B | 1/1 | ||||
| sYKL-40 | 1/1 | sIL-8 | 1/1 | ||||
| sAAT | 1/1 | sBSP | 1/1 | ||||
| sCAT-D | 1/1 | sfC6S/C4S | 1/1 | ||||
| sIL-10 | 1/1 | sCP-II | 1/1 | ||||
| sIL-12p70 | 1/1 | sICTP | 1/1 | ||||
| sKS | 1/1 | sfL2-KS | 1/1 | ||||
| sMMP-9 | 1/1 | sLeptin | 1/1 | ||||
| sNO | 1/1 | sTNF-α | 1/1 | ||||
| ssIL4-R | 1/1 | sfTRACP-5B | 1/1 |
OA = osteoarthritis; s25-OHVitd = serum25-hydroxyVitaminD; sAAT = serumalpha-1antitrypsin; sBSP = serumbonesialoprotein; sCAT-D = serumcathepsinD; sCOMP = serumcartilageoligomericprotein; sCP-II = serumC-propeptide; sCRP = serumC-reactiveprotein; sDCOMP = serumdeaminatedcartilageoligomericprotein; sICTP = serumcrosslinkingC-terminaltelopeptide; sIL = seruminterleukin; sIL-12p70 = seruminterleukin12p70; ssIL4-R = serumsolubleIL-4receptor; sKS = serumkeratansulfate; sLeptin = serumleptin; sMMP3 = serummatrixmetalloproteinase-3; sMMP-9 = serummatrixmetalloproteinase-9; sNO = serumnitrousoxide; sNTX = serumN-terminaltelopeptide; sTGF-B1 = serumtransforminggrowthfactorβ1; sTIMP-1 = serumTIMPmetalloproteinaseinhibitor1; sTNF-α = serumtumornecrosisfactor-alpha; sVCAM-1 = serumvascularcelladhesionmolecule1; sYKL-40 = serumhumancartilageglycoprotein39; sfC6S/C4S = synovialfluidchondroitin-6-sulfatetochondroitin-4-sulfateratio; sfFAC = serumfibronectin-aggrecancomplex; sfL2-KS = serumB-galactosyl-(1-4)-6-0-sulfo-N-acetylglucosamine(L2fragmentofkeratansulfate); sfTRACP-5B = serumtartrate-resistantacidphosphatase5b; uCTX-II = urinarytypeIIcollagentelopeptide; uDPD = urinarydeoxypyridinoline; uHelix-II = urinarytypeIIcollagenhelicaltelopeptide; uPYD = urinarypyridinoline.
Our study has several limitations. The use of systemic biomarkers (serum or urine) may be confounded by physiologic or pathologic processes in other joints with crossover on a given marker. Systemic biomarkers may also be subject to more daily variation in levels based on changes in time of day, activity level, and other factors. Use of biomarkers in synovial fluid is limited by the small amount of synovial fluid present and the difficulty in accessing the fluid, particularly in the clinical setting. Synovial fluid access at the time of surgery is more feasible but often requires a lavage, which limits the accuracy of biomarker concentration measurements [2]. Significant heterogeneity exists across different studies that can be difficult to adequately account for in a systematic review. Even for an individual biomarker, studies do not always agree; across studies, major differences in mean levels of the same biomarker level of cohorts with hip OA have been reported. The reason for such variation is difficult to determine but could include differences in assays used, disease severities studied, and patient populations evaluated. In general, most studies appear to be adequately powered to detect large effect sizes. Additionally, although our study summarizes the available literature, molecular biomarkers with evidence found in only a single study are not emphasized, but such biomarkers could prove to be valuable as supportive data from future studies become available.
The potential for molecular biomarkers to allow for diagnosis of early stages of hip OA has been investigated in a number of studies but remains inadequate for clinical use at this point. Sixteen biomarkers have demonstrated potential in this area (Table 2). Both urinary CTX-II (three of three studies) and serum CRP (two of three studies) demonstrated potential to differentiate between patients with hip OA and control subjects in more than one study. Urinary CTX-II is felt to be a biomarker specific to cartilage breakdown but is not however specific to the hip. Serum CRP on the other hand may have limited applicability in clinical use in hip disease as a result of effects of other underlying or transient conditions that may result in inflammation. Nonetheless, the number of studies demonstrating associations of hip OA and serum CRP suggests that the pathophysiology of hip OA does result in inflammation that can be detected on the systemic level. Although numerous studies in the “diagnosis” category demonstrate differences in mean levels between patients with hip OA and control subjects, further research is needed to demonstrate if any threshold biomarker levels are precise and specific enough to be useful clinically.
The ability of molecular biomarkers to serve as indicators of the severity of underlying hip degeneration is another promising future use of biomarkers. Similar to the “diagnosis” category, uCTX-II (two of two studies) and serum CRP (two of four studies) have the most evidence currently supporting this function. Although serum COMP demonstrated use in the “prognosis” category, only one of four studies demonstrated a difference in “disease staging”. Differences in study design do not clearly explain these differences; thus, the use of COMP remains to be seen. A recent study suggests that DCOMP may have more use in the pathophysiology of hip OA than COMP, but further research is needed. Although current literature focuses on varying stages of hip OA, future research investigations including the spectrum of prearthritic hip disease (including FAI and hip dysplasia) are important. Clinically useful biomarkers would need to reflect disease severity across this entire spectrum of the pathophysiology of hip OA.
The potential for level of a biomarker to estimate a patient’s prognosis is complex and relatively poorly investigated currently. Elevations in biomarker level that appear to affect prognosis may be confounded as markers of disease severity. Serum COMP is the only biomarker associated with OA prognosis in more than one study (three studies). However, the effect sizes seen in all three studies were relatively small (d ≤ 0.3) and one study [7] demonstrates contradicatory directions of significant associations questions the value of COMP in this setting. Urinary CTX-II was associated with OA prognosis in one study. Definitive studies in this area would need to correct for the baseline differences in disease severity based on biomarker level to make valid conclusions. Additionally, with the complicating role of surgical intervention in prognosis, “prognosis” will be difficult to investigate in the future.
Prearthritic hip disease presents the greatest potential for clinical use of biomarkers in early diagnosis, disease staging, and prognosis before the occurrence of irreversible articular cartilage damage, which is present once radiographic OA can be detected. Our improved understanding of prearthritic hip disease now includes the recognition of FAI and hip dysplasia as the two predominant causes of hip OA. Surgical treatment of these conditions before end-stage OA demonstrates clear differences in the pathophysiology of these conditions that both ultimately can lead to OA. The molecular biomarker profiles for these conditions are likely different and may account for heterogeneity in the current literature on biomarkers in hip OA that may contribute to the lack of identification of a clinically useful biomarker. The literature on biomarkers in prearthritic hip disease is limited with three studies investigating three different biomarkers [2, 4, 16] including two studies on FAI, one on pain of unspecified etiology and no studies investigated hip dysplasia. Bedi et al. [4] performed the best study to date in a small population with FAI and demonstrated differences in serum COMP and CRP compared with control subjects. Further investigations of these and additional biomarkers, particularly uCTX-II, in larger populations are needed. Additionally, investigations in populations with hip dysplasia are similarly needed. The application of the available literature on biomarkers, from established hip OA to future studies in FAI and hip dysplasia, may allow for rapid advancement of our understanding of biomarkers in this underinvestigated area of OA pathophysiology before the development of OA. Biomarker investigations in FAI and hip dysplasia should include the subgroup of biomarkers identified in our review as the most validated for hip OA and should similarly focus on study designs that will allow for advancement of our understanding of diagnosis, disease staging, and prognosis of prearthritic hip disease and, thus, hip OA.
In summary, although no current biomarker of hip OA has been adequately validated for clinical use, our systematic review found biomarkers exhibiting associations with hip OA in more than one study including uCTX-II, serum CRP, and serum COMP. Systemic biomarkers (currently used or developed in the future) continue to offer great potential for use in future clinical care but have some associated limitation. Future research is needed to establish the potential use of molecular biomarkers in a research or clinical setting. Future applications of associated biomarkers, from hip OA to prearthritic hip disease, including FAI and hip dysplasia, offer great promise for early diagnosis, disease staging, and treatment.
Electronic supplementary material
Acknowledgments
We thank Angela Hardi for her assistance with the literature review.
Footnotes
One or more of the authors certifies that he (JJN, JCC), or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of less than USD 10,000 USD from Smith & Nephew (JJN; Andover, MA, USA) and Biomet (JCC; Warsaw, IN, USA). The institution of one or more of the authors (JJN, JCC) has received, during the study period, funding from, Zimmer (JCC; Warsaw, IN, USA), Wright Medical (JCC; Memphis, TN, USA), Pivot Medical (JCC; Sunnyvale, CA, USA), and the Curing Hip Disease Fund (JCC). This publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (JJN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
References
- 1.Abe H, Sakai T, Ando W, Takao M, Nishii T, Nakamura N, Hamasaki T, Yoshikawa H, Sugano N. Synovial joint fluid cytokine levels in hip disease. Rheumatology (Oxford). 2014;53:165–172. doi: 10.1093/rheumatology/ket334. [DOI] [PubMed] [Google Scholar]
- 2.Abrams GD, Safran MR, Shapiro LM, Maloney WJ, Goodman SB, Huddleston JI, Bellino MJ, Scuderi GJ. Fibronectin-aggrecan complex as a marker for cartilage degradation in non-arthritic hips. Knee Surg Sports Traumatol Arthrosc. 2014;22:768–773. doi: 10.1007/s00167-014-2863-2. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Thers. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 4.Bedi A, Lynch EB, Sibilsky Enselman ER, Davis ME, Dewolf PD, Makki TA, Kelly BT, Larson CM, Henning PT, Mendias CL. Elevation in circulating biomarkers of cartilage damage and inflammation in athletes with femoroacetabular impingement. Am J Sports Med. 2013;41:2585–2590. doi: 10.1177/0363546513499308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger CE, Kroner A, Stiegler H, Leitha T, Engel A. Elevated levels of serum type I collagen C-telopeptide in patients with rapidly destructive osteoarthritis of the hip. Int Orthop. 2005;29:1–5. doi: 10.1007/s00264-004-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catterall JB, Hsueh MF, Stabler TV, McCudden CR, Bolognesi M, Zura R, Jordan JM, Renner JB, Feng S, Kraus VB. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem. 2012;287:4640–4651. doi: 10.1074/jbc.M111.249649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaganti RK, Kelman A, Lui L, Yao W, Javaid MK, Bauer D, Nevitt M, Lane NE. Change in serum measurements of cartilage oligomeric matrix protein and association with the development and worsening of radiographic hip osteoarthritis. Osteoarthritis Cartilage. 2008;16:566–571. doi: 10.1016/j.joca.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaganti RK, Parimi N, Cawthon P, Dam TL, Nevitt MC, Lane NE. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: the osteoporotic fractures in men study. Arthritis Rheum. 2010;62:511–514. doi: 10.1002/art.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevalier X, Conrozier T, Gehrmann M, Claudepierre P, Mathieu P, Unger S, Vignon E. Tissue inhibitor of metalloprotease-1 (TIMP-1) serum level may predict progression of hip osteoarthritis. Osteoarthritis Cartilage. 2001;9:300–307. doi: 10.1053/joca.2000.0389. [DOI] [PubMed] [Google Scholar]
- 10.Clohisy JC, Dobson MA, Robison JF, Warth LC, Zheng J, Liu SS, Yehyawi TM, Callaghan JJ. Radiographic structural abnormalities associated with premature, natural hip-joint failure. J Bone Joint Surg Am. 2011;93(Suppl 2):3–9. doi: 10.2106/JBJS.J.01734. [DOI] [PubMed] [Google Scholar]
- 11.Conrozier T, Carlier MC, Mathieu P, Colson F, Debard AL, Richard S, Favret H, Bienvenu J, Vignon E. Serum levels of YKL-40 and C reactive protein in patients with hip osteoarthritis and healthy subjects: a cross sectional study. Ann Rheum Dis. 2000;59:828–831. doi: 10.1136/ard.59.10.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrozier T, Chappuis-Cellier C, Richard M, Mathieu P, Richard S, Vignon E. Increased serum C-reactive protein levels by immunonephelometry in patients with rapidly destructive hip osteoarthritis. Rev Rhum Engl Ed. 1998;65:759–765. [PubMed] [Google Scholar]
- 13.Conrozier T, Ferrand F, Poole AR, Verret C, Mathieu P, Ionescu M, Vincent F, Piperno M, Spiegel A, Vignon E. Differences in biomarkers of type II collagen in atrophic and hypertrophic osteoarthritis of the hip: implications for the differing pathobiologies. Osteoarthritis Cartilage. 2007;15:462–467. doi: 10.1016/j.joca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Conrozier T, Merle-Vincent F, Mathieu P, Richard M, Favret H, Piperno M, Caton J, Vignon E. Epidemiological, clinical, biological and radiological differences between atrophic and hypertrophic patterns of hip osteoarthritis: a case-control study. Clin Exp Rheumatol. 2004;22:403–408. [PubMed] [Google Scholar]
- 15.Conrozier T, Saxne T, Fan CSS, Mathieu P, Tron AM, Heinegard D, Vignon E. Serum concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: a one year prospective study. Ann Rheum Dis. 1998;57:527–532. doi: 10.1136/ard.57.9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragomir AD, Kraus VB, Renner JB, Luta G, Clark A, Vilim V, Hochberg MC, Helmick CG, Jordan JM. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002;10:687–691. doi: 10.1053/joca.2002.0816. [DOI] [PubMed] [Google Scholar]
- 17.Engström G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009;17:168–173. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Garnero P, Charni N, Juillet F, Conrozier T, Vignon E. Increased urinary type II collagen helical and C telopeptide levels are independently associated with a rapidly destructive hip osteoarthritis. Ann Rheum Dis. 2006;65:1639–1644. doi: 10.1136/ard.2006.052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnero P, Conrozier T, Christgau S, Mathieu P, Delmas PD, Vignon E. Urinary type II collagen C-telopeptide levels are increased in patients with rapidly destructive hip osteoarthritis. Ann Rheum Dis. 2003;62:939–943. doi: 10.1136/ard.62.10.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnero P, Mazières B, Guéguen A, Abbal M, Berdah L, Lequesne M, Nguyen M, Salles JP, Vignon E, Dougados M. Cross-sectional association of 10 molecular markers of bone, cartilage, and synovium with disease activity and radiological joint damage in patients with hip osteoarthritis: the ECHODIAH cohort. J Rheumatol. 2005;32:697–703. [PubMed] [Google Scholar]
- 21.Hulejová H, Barešová V, Klézl Z, Polanská M, Adam M, Šenolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007;38:151–156. doi: 10.1016/j.cyto.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Jung M, Christgau S, Lukoschek M, Henriksen D, Richter W. Increased urinary concentration of collagen type II C-telopeptide fragments in patients with osteoarthritis. Pathobiology. 2004;71:70–76. doi: 10.1159/000074419. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki M, Hasegawa Y, Kondo S, Iwata H. Concentration and localization of YKL-40 in hip joint diseases. J Rheumatol. 2001;28:341–345. [PubMed] [Google Scholar]
- 24.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of higher levels of serum cartilage oligomeric matrix protein and N-telopeptide crosslinks with the development of radiographic hip osteoarthritis in elderly women. Arthritis Rheum. 2006;54:236–243. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 25.Kenanidis E, Potoupnis ME, Papavasiliou KA, Pellios S, Sayegh FE, Petsatodis GE, Karatzas N, Kapetanos GA. The serum levels of receptor activator of nuclear factor-kappaB ligand, bone-specific alkaline phosphatase, osteocalcin and osteoprotegerin do not correlate with the radiographically assessed severity of idiopathic hip and knee osteoarthritis. Clin Biochem. 2011;44:203–207. doi: 10.1016/j.clinbiochem.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73:423–429. doi: 10.1302/0301-620X.73B3.1670443. [DOI] [PubMed] [Google Scholar]
- 27.Koorts AM, Levay PF, Hall AN, van der Merwe CF, Becker PJ, Frantzen DJM, Viljoen M. Expression of the H- and L-subunits of ferritin in bone marrow macrophages of patients with osteoarthritis. Exp Biol Med. 2012;237:688–693. doi: 10.1258/ebm.2012.011278. [DOI] [PubMed] [Google Scholar]
- 28.Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, Gardiner J, Garnero P, Hardin J, Henrotin Y, Heinegard D, Ko A, Lohmander LS, Matthews G, Menetski J, Moskowitz R, Persiani S, Poole AR, Rousseau JC, Todman M. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19:515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane NE, Gore LR, Cummings SR, Hochberg MC, Scott JC, Williams EN, Nevitt MC. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42:854–860. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Lane NE, Nevitt MC, Lui LY, de Leon P, Corr M. Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly Caucasian women. Arthritis Rheum. 2007;56:3319–3325. doi: 10.1002/art.22867. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77:985–989. doi: 10.2106/00004623-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38:810–824. doi: 10.1259/0007-1285-38-455-810. [DOI] [PubMed] [Google Scholar]
- 34.Nelson AE, Fang F, Shi XA, Kraus VB, Stabler T, Renner JB, Schwartz TA, Helmick CG, Jordan JM. Failure of serum transforming growth factor-beta (TGF-beta1) as a biomarker of radiographic osteoarthritis at the knee and hip: a cross-sectional analysis in the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2009;17:772–776. doi: 10.1016/j.joca.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson AE, Golightly YM, Kraus VB, Stabler T, Renner JB, Helmick CG, Jordan JM. Serum transforming growth factor-beta 1 is not a robust biomarker of incident and progressive radiographic osteoarthritis at the hip and knee: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2010;18:825–829. doi: 10.1016/j.joca.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olszewska-Slonina D, Matewski D, Jung S, Olszewski KJ, Czajkowski R, Braszkiewicz J, Wozniak A, Kowaliszyn B. The activity of cathepsin D and alpha-1 antitrypsin in hip and knee osteoarthritis. Acta Biochim Pol. 2013;60:99–106. [PubMed] [Google Scholar]
- 37.Reijman M, Hazes JMW, Bierma-Zeinstra SMA, Koes BW, Christgau S, Christiansen C, Uitterlinden AG, Pols HAP. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50:2471–2478. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 38.Salvatierra J, Escames G, Hernandez P, Cantero J, Crespo E, Leon J, Salvatierra D, Acuna-Castroviejo D, Vives F. Cartilage and serum levels of nitric oxide in patients with hip osteoarthritis. J Rheumatol. 1999;26:2015–2017. [PubMed] [Google Scholar]
- 39.Schett G, Kiechl S, Bonora E, Zwerina J, Mayr A, Axmann R, Weger S, Oberhollenzer F, Lorenzini R, Willeit J. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. 2009;60:2381–2389. doi: 10.1002/art.24757. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri T, Pulsatelli L, Dolzani P, Facchini A, Meliconi R. Elevated serum levels of soluble interleukin-4 receptor in osteoarthritis. Osteoarthritis Cartilage. 2006;14:717–719. doi: 10.1016/j.joca.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58:176–183. doi: 10.1302/0301-620X.58B2.932079. [DOI] [PubMed] [Google Scholar]
- 42.Stannus OP, Jones G, Quinn SJ, Cicuttini FM, Dore D, Ding C. The association between leptin, interleukin-6, and hip radiographic osteoarthritis in older people: a cross-sectional study. Arthritis Res Ther. 2010;12:R95. doi: 10.1186/ar3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart A, Black A, Robins SP, Reid DM. Bone density and bone turnover in patients with osteoarthritis and osteoporosis. J Rheumatol. 1999;26:622–626. [PubMed] [Google Scholar]
- 44.Sweet MBE, Coelho A, Schnitzler CM, Schnitzer TJ, Lenz ME, Jakim I, Kuettner KE, Thonar EJMA. Serum keratan sulfate levels in osteoarthritis patients. Arthritis Rheum. 1988;31:648–652. doi: 10.1002/art.1780310510. [DOI] [PubMed] [Google Scholar]
- 45.van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FP. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18:605–612. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Vos PA, DeGroot J, Huisman AM, Oostveen JC, Marijnissen AC, Bijlsma JW, van El B, Zuurmond AM, Lafeber FP. Skin and urine pentosidine weakly correlate with joint damage in a cohort of patients with early signs of osteoarthritis (CHECK) Osteoarthritis Cartilage. 2010;18:1329–1336. doi: 10.1016/j.joca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Yamada H, Miyauchi S, Hotta H, Morita M, Yoshihara Y, Kikuchi T, Fujikawa K. Levels of chondroitin sulfate isomers in synovial fluid of patients with hip osteoarthritis. J Orthop Sci. 1999;4:250–254. doi: 10.1007/s007760050100. [DOI] [PubMed] [Google Scholar]
- 48.Yamada H, Miyauchi S, Morita M, Yoshida Y, Yoshihara Y, Kikuchi T, Washimi O, Washimi Y, Terada N, Seki T, Fujikawa K. Content and sulfation pattern of keratan sulfate in hip osteoarthritis using high performance liquid chromatography. J Rheumatol. 2000;27:1721–1724. [PubMed] [Google Scholar]
- 49.Yamaguchi R, Yamamoto T, Motomura G, Ikemura S, Iwasaki K, Zhao G, Doi T, Iwamoto Y. Bone and cartilage metabolism markers in synovial fluid of the hip joint with secondary osteoarthritis. Rheumatology (Oxford) 2014;53:2191–2195. doi: 10.1093/rheumatology/keu253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.