Abstract
Tools pose a challenge to the need to select actions appropriate for task goals and environmental constraints. For many tools (e.g., calculator), actions for “using” and “grasping-to-move” conflict with each other and may compete during selection. To date, little is known about the mechanisms that enable selection between possible tool actions or their neural substrates. The study of patients with chronic left hemisphere stroke, many of whom are deficient in tool-use action (apraxic), provides an opportunity to elucidate these issues. Here, 31 such patients pantomimed or recognized tool use actions for “conflict” and “non-conflict” tools. Voxel-based lesion-symptom mapping, lesion subtraction, and tractographic overlap analyses were used to determine brain regions necessary for selecting among tool-directed actions. Lesions to posterior middle temporal gyrus (pMTG) and anterior intraparietal sulcus (aIPS) tended to impair production of use actions similarly for both conflict and non-conflict tools. By contrast, lesions to the supramarginal gyrus (SMG), inferior frontal gyrus (IFG)/anterior insula, and superior longitudinal fasciculus (SLF) specifically impaired production of use actions for conflict tools. Patients' errors on conflict tools suggested inappropriate selection of grasping actions and difficulty selecting single actions. Use/grasp conflict had no effect on action recognition. We suggest that the SMG/SLF/IFG pathway implements biased competition between possible tool actions, while aIPS and pMTG compute the structure-based and skilled use actions, respectively, that constitute input to this competitive process. This is the first study to demonstrate a reliable link between a characteristic of single tools (i.e., their association with different use and grasp actions) and action selection difficulties. Additionally, the data allow us to posit a SMG-involved subtype of apraxia characterized by an inability to resolve action competition.
Keywords: action selection, tool use, inferior parietal lobe, apraxia, VLSM
1. Introduction
A fundamental problem for the brain is the specification of potential actions and the need to select among these actions according to task goals. Substantial research indicates that the sensorimotor system prepares possible actions in parallel while awaiting additional information required to select between them (e.g., Cisek & Kalaska, 2005; Kim & Shadlen, 1999; Ledberg, Bressler, Ding, Coppola, & Nakamura, 2007; Pastor-Bernier & Cisek, 2011; see Cisek & Kalaska, 2010; Gold & Shadlen, 2007 for reviews). As evidence for each action accumulates, candidate actions compete with one another for selection, and selection is biased in favor of actions consistent with context and goals (Cisek, 2007).
For humans, interacting with tools poses a special challenge for action selection: many tools can be used with more than one skilled action (e.g., a knife can be used for slicing, stabbing, or spreading). Furthermore, for some tools, actions associated with skillful use differ from actions for transport. For example, a calculator is used with a non-prehensile “poke”, but it is picked up and moved with a power grip. In fact, “grasp-to-move” and “use” actions are associated with different temporal dynamics of activation. While grasp-to-move actions are rapidly evoked but short-lasting, use actions show comparatively slower activation and decay (Jax & Buxbaum, 2010; Lee, Middleton, Mirman, Kalenine, & Buxbaum, 2012). Because of these differences in the time-course of their activation, grasp actions may interfere with use actions within single tools (Jax & Buxbaum, 2010; Osiurak, Roche, Ramone, & Chainay, 2013). For example, Jax & Buxbaum (2010) found that participants were slower to initiate use actions to tools associated with different use and grasp actions (e.g., calculator) than to tools associated with the same use and grasp actions (e.g., beer mug). These results indicate that an inconsistent grasp action can interfere with the production of a tool use action. However, no such effect was observed when participants initiated grasp actions (that is, a different use did not interfere with grasping), unless they had completed a use task prior to grasping. These and other related data (e.g., Lee et al., 2012) indicate that interference from use actions on grasping takes longer to emerge and may arise during the retrieval and processing of semantic knowledge of tools. In contrast, grasp actions are more quickly computed, based on currently–visualized structural properties of objects, and so grasp can interfere with use even on an individual trial, within single objects. In light of these data, a critical question is what mechanisms—and which brain regions—enable selection of appropriate tool-related hand actions.
An important opportunity to examine this issue is afforded by studying the determinants and neuroanatomic substrates of errors in patients with limb apraxia, a disorder of skilled action characterized by spatiotemporal and postural hand action errors. Patients with apraxia after left hemisphere stroke (LCVA) exhibit slowed activation of “use” actions (Lee, Mirman, & Buxbaum, 2014), and, relative to control participants and non-apraxic patients, erroneously grasp (and subsequently erroneously use) tools when asked to use them but not when asked to transport them (Randerath, Li, Goldenberg, & Hermsdörfer, 2009). Furthermore, patients with apraxia have particular difficulty producing hand actions for tools associated with conflicting use and grasp actions, like a calculator (“conflict” tools) (Jax & Buxbaum, 2013). Even so, these patients perform normally when reaching and/or generating grasping actions based on object shape and size (Buxbaum, Johnson-Frey, & Bartlett-Williams, 2005; Buxbaum, Sirigu, Schwartz, & Klatzky, 2003; Haaland, Harrington, & Knight, 1999). In contrast to patients with limb apraxia, patients with optic ataxia exhibit impairments when grasping objects but can often correctly pantomime object use actions (Karnath & Perenin, 2005; Perenin & Vighetto, 1988).
This pattern of data suggests that functionally and/or neuroanatomically distinct cognitive systems subserve skilled use of tools and prehensile grasping. In addition, neuroimaging studies of healthy participants reveal different patterns of activation for these two kinds of actions with objects (Buxbaum, Kyle, Tang, & Detre, 2006; Creem-Regehr, Dilda, Vicchrilli, Federer, & Lee, 2007). Although visually-guided control of action relies on brain regions in the dorsal processing stream (Goodale & Milner, 1992; Goodale, Milner, Jakobson, & Carey, 1991), several researchers have proposed further divisions of the dorsal stream for different kinds of object-directed actions (Binkofski & Buxbaum, 2013; Buxbaum & Kalenine, 2010; Fridman et al., 2006; Johnson-Frey, 2004; Rizzolatti & Matelli, 2003; Vingerhoets, Acke, Vandemaele, & Achten, 2009). Specifically, a bilateral dorso-dorsal “Grasp” system is specialized for prehensile actions based on object shape, size, and orientation, while a left-lateralized ventro-dorsal “Use” system mediates skilled object use actions that cannot be inferred from object structure.
The decision to use a tool or grasp it to move depends on context and task goals. Moreover, everyday actions often entail both moving and using in relatively rapid succession (e.g., when selecting a tool from a drawer or storage container, performing a task with the tool, and then clearing it from the workspace) and likely require coordination between Use and Grasp systems (Binkofski & Buxbaum, 2013). Yet, little is known about how different actions specified by these two systems compete for selection. Many important questions remain, including which regions within the left hemisphere normally select between tool-directed actions, the impact of deficient selection on apraxic errors, and the stage of cognitive processing at which such errors arise.
Neuroimaging studies implicate left inferior gyrus (IFG)/ventral premotor cortex (vPMC), inferior parietal cortex (IPL), and posterior middle temporal gyrus (pMTG) as key nodes in the network subserving skilled tool use (Lewis, 2006), and lesions to each of these regions are associated with apraxia (Buxbaum, Shapiro, & Coslett, 2014; Randerath, Goldenberg, Spijkers, Li, & Hermsdörfer, 2010). Two of these regions—IFG and IPL—may play a role in selection, broadly defined. On many accounts, IFG resolves competition that arises when selecting between incompatible representations (e.g., Thompson-Schill & Botvinick, 2006). Similarly, anterior parietal cortex/supramarginal gyrus (SMG) is activated during response competition (Hazeltine, Poldrack, & Gabrieli, 2000) and may update or suppress prepared but incorrect actions (Hartwigsen et al., 2012). However, studies of response conflict typically examine simple and/or arbitrary actions (e.g., button presses) with questionable relevance to tool actions.
In the present study, we used voxel-based lesion-symptom mapping (VLSM) with LCVA patients to test the hypothesis that within the key nodes of the tool-use network, IFG and SMG (but not pMTG) enable selection between different hand actions naturally associated with the same tool. While apraxia is apparent in actual tool use (e.g., Poizner, Mack, Verfaellie, Gonzalez Rothi, & Heilman, 1990), object structure constrains the degrees of freedom of movements (see Buxbaum, Johnson-Frey, et al., 2005). Consequently, we assessed performance using tool use pantomime since it is correlated with tool use (Jarry et al., 2013), is more likely to reveal subtle influences on apraxic performance (Buxbaum, Kyle, & Menon, 2005), and results in movement errors similar in character to those seen with tool use (Hermsdörfer, Li, Randerath, Roby-Brami, & Goldenberg, 2013). Additionally, we confirmed that the effects of deficient action selection are evident in action production but not in a task that merely requires recognition of tool use actions (tool use pantomime recognition). Finally, we tested the prediction that an inability to select between use and grasp actions results in inappropriate grasping responses (due to the relative preservation of the Grasp system in patients with limb apraxia, Jax & Buxbaum, 2013) and/or difficulty selecting a single response. The results of this study enable us to provide both computational and neuroanatomic specificity to our understanding of action selection.
2. Materials & Methods
2.1 Participants
We recruited 31 chronic left hemisphere stroke patients (48% female) from the Neuro-Cognitive Rehabilitation Research Registry at Moss Rehabilitation Research Institute (MRRI) (Schwartz, Brecher, Whyte, & Klein, 2005) (48% female; mean age = 57.0 years, SD = 10.6, range = 31–76 years; mean education = 15.7 years, SD = 1.5, range = 11–29 years). All patients were at least 6 months post-stroke. To ensure that patients understood instructions for the experimental tasks, we excluded patients with severe language comprehension deficits, defined as scores of 4 or lower on the comprehension subtest of the Western Aphasia Battery (Kertesz, 1982). Demographic, neuropsychological, and lesion information for all LCVA patients are reported in Table 1. Note that patients were not specifically selected for the presence of limb apraxia; rather, the behavioral and neuroanatomic methods we employed were predicated upon a full range of performance. Similarly, we did not categorize patients as having different types of apraxia (e.g., ideomotor or ideational apraxia). Historically, definitions of ideomotor and ideational apraxia have varied across laboratories (Buxbaum, 2001), and correlations between tasks that measure the underlying deficits for each type (e.g., Buxbaum, Kyle, et al., 2005) suggest that “pure” ideomotor and ideational apraxia are rare. Consequently, we use the generic term “apraxia” to describe patients in this study, where applicable.
Table 1.
Demographic, neuropsychological, and lesion information for left hemisphere stroke patients.
| Patient | Age (years) | Gender | Handedness | Education (years) | WABC | Lesion volume (mm)3 |
|---|---|---|---|---|---|---|
| 1 | 58 | female | right | 18 | 6.9 | 213473 |
| 2 | 64 | male | right | 12 | 7.2 | 188265 |
| 3 | 58 | male | right | 16 | 8.5 | 100822 |
| 4 | 51 | female | right | 16 | 8.1 | 143097 |
| 5 | 43 | female | right | 13 | 9.2 | 48947 |
| 6 | 48 | female | right | 16 | 9.4 | 81853 |
| 7 | 54 | male | right | 13 | 9.9 | 162349 |
| 8 | 68 | male | right | 19 | 9.4 | 83120 |
| 9 | 53 | male | right | 19 | 6.8 | 210758 |
| 10 | 76 | male | right | 21 | 8.9 | 7986 |
| 11 | 74 | male | right | 29 | 8.9 | 35702 |
| 12 | 54 | male | right | 18 | 9.8 | 81641 |
| 13 | 62 | female | right | 16 | 8.7 | 33759 |
| 14 | 53 | female | right | 14 | 9.4 | 30932 |
| 15 | 50 | male | right | 14 | 9.5 | 5347 |
| 16 | 48 | female | right | 13 | 9.2 | 79640 |
| 17 | 66 | male | both | 16 | 4.8 | 228749 |
| 18 | 59 | female | right | 16 | 9.5 | 193865 |
| 19 | 56 | male | right | 12 | 8.7 | 55800 |
| 20 | 62 | female | right | 16 | 10.0 | 46167 |
| 21 | 60 | male | right | 15 | 9.0 | 190376 |
| 22 | 57 | male | right | 12 | 9.8 | 23076 |
| 23 | 60 | female | right | 16 | 9.6 | 72212 |
| 24 | 67 | male | right | 14 | 4.6 | 65573 |
| 25 | 33 | female | right | 19 | 6.6 | 63241 |
| 26 | 72 | female | right | 11 | 8.7 | 50553 |
| 27 | 73 | female | right | 14 | 9.5 | 9066 |
| 28 | 49 | male | right | 14 | 8.5 | 53231 |
| 29 | 52 | female | right | 18 | 7.5 | 6795 |
| 30 | 55 | female | right | 12 | 10.0 | 126490 |
| 31 | 31 | male | right | 13 | 5.7 | 87250 |
Note: WABC refers to the number of correct responses (out of 10) on the comprehension subtest of the Western Aphasia Battery.
In addition to this group of patients, we recruited neurologically-intact control participants from the Control Subject Research Registry at MRRI. All control participants were right-handed and achieved a minimum score of 27/30 on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975). Initially, fifteen control participants were administered the experimental tasks. However, due to an administration error, these control participants completed the pantomime task using the right hand. (All patients pantomimed using the less-impaired left hand.) Therefore, we re-administered the pantomime task to a subset (n = 12) of these controls after a delay of at least 19 months (two of the fifteen controls were unavailable, and one was excluded for having a MMSE score less than 27/30 upon re-test). To increase the number of control participants, we also administered the pantomime task to seven new control participants1. One was excluded for not following instructions, a second was excluded for abnormally low pantomime accuracy (more than 2.5 standard deviations below the control group mean), and a third was excluded because 20% of trials were performed outside of the camera frame. Thus, sixteen control participants served as the final control group for the pantomime task (75% female; mean age = 64.4 years, SD = 9.9, range = 45–78 years; mean education = 16.7 years, SD = 2.9; range = 12–21 years). Participants in the pantomime control group did not differ from the patient group on years of education [t(45) = .97, p = .34] but were significantly older [t(45) = 2.31, p = .03].
The recognition control group consisted of the fifteen participants initially administered both experimental tasks, as described above; one of these participants was subsequently excluded for having a low MMSE score upon re-testing. Participants in the final recognition control group (n = 14; 64% female; mean age = 63.1 years, SD = 9.8, range = 48–78 years; mean education = 17.0 years, SD = 2.1, range = 12–20 years) did not differ on years of education from the patients [t(43) = 1.29, p = .20] but were also marginally older [t(43) = 1.83, p = .07]. These differences in age for both pantomime and recognition control groups were likely due to the presence of two younger LCVA patients (ages 31 and 33). Note that given that performance on many cognitive tasks decreases with age, the older control groups work against the hypothesis that patients are more deficient than controls.
Any participant with a history of co-morbid or pre-morbid neurologic disorders, alcohol or drug abuse, or psychosis was excluded from the study. All participants gave informed consent to participate in the behavioral portion of the experiment in accordance with the guidelines of the Institutional Review Board of the Albert Einstein Healthcare Network and were paid for their participation. In addition, 29 of the 31 LCVA patients provided informed consent to participate in a structural magnetic resonance imaging (MRI) or computed tomography (CT) protocol at the University of Pennsylvania; brain scans for the remaining 2 patients were obtained from clinical records (see below). Participants were paid for their participation in this portion of the experiment and reimbursed for travel expenses.
2.2 Stimuli
2.2.1 Stimuli for pantomime and recognition tasks
We focused our inquiry on pantomimed actions to the sight of tools and recognition of tool use actions, given the sensitivity and frequency with which these tasks are used diagnose apraxia (Rothi, Raymer, & Heilman, 1997). In both experimental tasks, we used as stimuli or response options color photographs of tools selected from the 480 photographs of the Bank of Standardized Stimuli (BOSS) (Brodeur, Dionne-Dostie, Montreuil, & Lepage, 2010). To select these stimuli, we first identified photographs that depicted manipulable artifacts associated with distinct use actions (i.e., tools). These objects included construction tools (e.g., hammer), household articles (e.g., sponge), school supplies (e.g., scissors), kitchen items (e.g., wooden spoon), and bathroom items (e.g., razor). None of the photographs depicted living things, such as plants or food, and all tools could be used with one hand.
2.2.1.1 Normative ratings
With this pool of photographs, we conducted a separate normative study in which 14 neurologically-intact adult participants (none of whom participated in the experiment) rated the tools for use/grasp conflict; they determined, on a 1 to 5 scale, “the extent to which the hand movements that you make to use the object differ from the hand movements that you make to pick it up”. These participants also rated the strength of the affordance associated with a tool (i.e., “the degree to which the shape of the object implies how it should be used”) on a scale from 1 to 5. Average name agreement and familiarity of the tool photographs were retrieved from the BOSS database (Brodeur et al., 2010). Using this normative data, we selected 20 “conflict” tools, i.e., tools used and grasped with different hand movements, and 20 “non-conflict” tools, i.e., tools used and grasped with the same hand movement (Figure 1). Subsequently, based on the performance of the control group on the pantomime task, we excluded two conflict tools for which accuracy was more than 2.5 standard deviations below the average for that tool type and pantomime component (see below). Thus, the final stimulus set contained 18 conflict tools and 20 non-conflict tools. These tools differed significantly on average conflict rating [t(36) = 9.43, p < .0005] but did not differ in terms of affordance strength [t(36) = 1.60, p = .12], name agreement [t(36) = .84, p = .41], or familiarity [t(36) = 1.34, p = .19]. These stimuli were used in both the pantomime and recognition tasks.
Figure 1.

Examples of photographs used in the experimental tasks: A) tools used and grasped with the same hand actions (“non-conflict” tools), and B) tools used and grasped with different hand actions (“conflict” tools).
2.2.1.2 Post-hoc normative ratings
At the suggestion of a reviewer, we also collected an additional rating for each tool to assess mechanical complexity. Tools with greater mechanically complexity may afford more potential actions, thus increasing competition during action selection. In these ratings, we used “moveable parts” as a proxy for mechanical complexity based on piloting suggesting that the former was assessed more reliably. If conflict tools tend to have more moveable parts, accuracy differences between conflict and non-conflict tools cannot be specifically attributed to use/grasp conflict. To control for this potential confound, we asked 10 neurologically-intact adult participants (none of whom participated in the experiment) to count, for each tool, the “number of moveable parts that you manipulate directly when using the object”2. Each tool received a rating of 0, 1, 2, or “greater than 2” moveable parts. For tools that received ratings of 0, 1, or 2 moving parts (n = 20), these ratings were averaged together.
We then used these moveable parts ratings—as well as ratings for affordance strength, name agreement, and familiarity—to select a subset of conflict and non-conflict tools that were closely matched on each of these dimensions. Conflict tools (n = 10) and non-conflict tools (n = 10) in this subset again differed significantly on average conflict rating [t(18) = 6.60, p < .0005] but were very well equated on affordance strength [t(18) = .52, p = .61], name agreement [t(18) = .53, p = .60], familiarity [t(18) = .46, p = .65], and average number of moveable parts [t(18) = .82, p = .42]. We then used this well-matched set of tools, in addition to the original set, to confirm the study hypotheses (see Section 3.1.4).
2.2.2 Additional stimuli for recognition task
In addition, for the recognition task, we created videos of tool use pantomimes that depicted an experimenter seated, facing the camera, pantomiming the use of each of the 40 tools with her right hand. We also developed sets of 4 tool photographs to be displayed on the computer screen after the pantomime videos. One of these 4 photographs was the target, selected from the 40 conflict and non-conflict tool photographs described above (e.g., for the video depicting a pantomime of “hammering”, the correct photograph was a hammer). The remaining 3 foil photographs in each set were selected from multiple sources (3 × 40 = 120 foils), and none of the 40 target items served as foils. Normative data collected from 13 neurologically-intact adult participants (none of whom participated in the experiment) ensured that foils for conflict (excluding two outlier tools, as described above) and non-conflict tools were equivalently similar to the target photograph on several dimensions: average pairwise visual similarity [t(36) = .37, p = .71], taxonomic/categorical relatedness [t(36) = .34, p = .74], thematic relatedness [t(36) = .71, p = .48], and similarity of use gestures [t(38) = .89, p = .38].
2.2 Procedure
Most participants completed the two experimental tasks as part of a larger set of tests that also included tool naming and tool name recognition. However, control participants newly recruited to perform the pantomime task with the left hand did not complete these other tasks. To focus our hypotheses on the nature of action selection, we only examine tool use pantomime and tool use recognition tasks here. For all patients (n = 31), the order in which these two tasks occurred was counter-balanced across patients. For the initial group of control participants, the order in which these tasks occurred was also counter-balanced. The remaining control participants (re-tested or newly recruited) completed the pantomime task on a separate day of testing. Stimuli for all tasks were presented on a 1680 × 1050 pixel screen using E-Prime (Psychology Software Tools, Pittsburgh, PA).
2.2.1 Pantomime task
On each trial of the tool use pantomime task, participants saw a 600 × 600 pixel color photograph of a tool on the screen. Participants were instructed to “show how you would use the object”. Given the possibility of right hemiparesis after left hemisphere stroke, patients pantomimed using their left hand, and control participants did the same. Participants completed 4 practice trials with feedback. Following Rothi & colleagues (1991), if a participant pantomimed the action as if his or her hand was the tool itself (e.g., positioning fingers as if they were the teeth of a comb), the participant was reminded to “show how you would use the object as if you are actually holding it in your hand”. Participants were corrected only the first time a “body-part-as-object” (BPO) error was made. If a participant said that he/she did not recognize a tool on the screen, the experimenter advanced to the next trial.
2.2.1.1 Accuracy coding
Pantomimes were recorded by video camera and scored offline for accuracy by a trained coder who obtained at least 85% agreement with previous coders in our lab (Buxbaum, Kyle, et al., 2005). Each pantomime was scored on 5 different dimensions (one semantic and four spatiotemporal) according to a detailed error taxonomy long in use in our laboratory (see Buxbaum, Giovannetti, & Libon, 2000; Buxbaum, Kyle, Grossman, & Coslett, 2007 for more details). First, each pantomime was given credit for semantic content unless a participant performed a recognizable gesture appropriate for using a semantically-related tool (e.g., sawing instead of hammering). Only pantomimes that received credit for content were scored on the remaining 4 components. Trials on which content errors occurred were very rare (.57% of patient trials); control participants made no content errors whatsoever. Twelve of 31 patients made at least one content error, and of those, 7 patients made only 1 content error. Therefore, content errors were not produced by sufficient numbers of participants to be considered for further analysis. Additionally, we did not score content errors for spatiotemporal accuracy since the semantically-related gesture produced as a content error, by definition, are incorrect on the four spatiotemporal dimensions (hand action, arm action, amplitude, and timing). Thus, for example, a clearly recognizable sawing gesture has the “wrong” arm action, amplitude, and timing for the target gesture of hammering, but the “source” of that error is assumed not to be spatiotemporal. Therefore, for content errors, we did not assign values to the spatiotemporal portions of the score. Furthermore, although participants were instructed to inform the experimenter if they did not recognize an object, we cannot exclude the possibility that some content errors reflect deficient object recognition. Note that we use a more restricted definition of content errors than some other studies. For instance, in the Florida Apraxia Battery (Rothi et al., 1991), content errors can be semantically related pantomimes (e.g., sawing instead of hammering), non-related pantomimes (e.g., playing a trombone instead of hammering), or perseverations of previously produced pantomimes. Thus, we report a lower rate of content errors than studies that use this broader definition (Hanna-Pladdy et al., 2001).
Scoring on the remaining 4 components (hand action, arm action, amplitude, and timing) was lenient, and credit was given unless errors were flagrant or performance was only transiently correct. A hand action error was assigned if the shape or movement trajectory of the hand and/or wrist was unrecognizable, flagrantly incorrect, or only transiently correct, or if the hand was used as part of the object. The latter were classified as body-part-as-object (BPO) errors (e.g., using the fingers as the teeth of a comb); see below. An arm action error was assigned if arm action and/or arm movement trajectory was flagrantly incorrect or only transiently correct. An amplitude error was assigned if the size of the movement was flagrantly too large or too small. A timing error was assigned if the speed of the movement was flagrantly too fast or too slow, or if the number of cycles of the movement were flagrantly too few or too many. For each participant, total pantomime scores for each item were calculated by averaging the 4 component scores.
To specifically determine the degree to which each patient was disproportionately impaired at producing hand actions for conflict relative to non-conflict tools, we regressed conflict tool hand action accuracy on non-conflict tool hand action accuracy. The standardized residual scores from this analysis represent the degree to which each patient's ability to produce the hand action for conflict tools was better or worse than expected given his or her performance on non-conflict tools. These standardized residuals were used to determine the relationship between apraxia severity (defined by overall accuracy on the pantomime task) and the effects of use/grasp conflict, as well as the lesion sites associated with disproportionately poor performance on conflict tools.
2.2.1.2 Hand action error coding
As noted, conflict tools were denoted based on a difference between the hand actions for using versus grasping-to-move. Accordingly, we expected the effects of use/grasp conflict to be most strongly apparent in the hand action component of pantomime. Moreover, prior studies have demonstrated that apraxics are most impaired in the hand action component of pantomime tasks (e.g., Buxbaum, Kyle, et al., 2005). To assess the hypothesis that an inability to select between candidate grasp and use actions would result in inappropriate grasping responses (Jax & Buxbaum, 2013) and/or failure to produce a single response, two trained coders who achieved 90% agreement performed a detailed coding of the type of hand action errors made by patients.
We tallied all hand action errors for both conflict and non-conflict tools and determined that, with seven error categories, we could adequately characterize the observed range of behavior for both tool types. Four error types reflected categorically inappropriate hand actions for use (e.g., making a “poke” gesture for a knife). These errors were “prehensile” (pinch, clench, flat clench), “non-prehensile” (poke, palm), a prehensile/non-prehensile “hybrid” (hand is clenched for grasping, but the thumb pokes/presses, e.g., to use a lighter), or a BPO error. The fifth type, “same as use” errors, were those reflecting incorrect execution of the appropriate hand action for use (e.g., a clench substantially too wide to accommodate the pictured exemplar of iron). Sixth, if the patient attempted multiple hand actions during a pantomime, the hand action was coded as a “multiple attempt” error. Finally, if the hand action error could not be assigned to any of these categories, it was coded as “other”. Analyses were performed on the percentage of errors of each type to conflict (n = 18) and non-conflict tools (n = 20).
2.2.2 Recognition task
On each trial of the tool use recognition task, participants viewed a video of an experimenter pantomiming the use of a tool. After the video completed, it was played a second time. Immediately thereafter, 4 colored tool photographs (each 450 × 450 pixels) appeared on the screen, arranged in a square, and participants were instructed to point to the tool whose use had been pantomimed in the video. The tool photographs remained on the screen until the participant made a response. The experimenter recorded the participant's response on the keyboard. Before the experimental trials, participants completed 3 practice trials with feedback.
2.3 Imaging Methods
2.3.1 Image acquisition
For patients, we acquired structural brain images. Twenty-nine patients received research-quality MRI or CT scans. For 19 of 29 patients, we collected high-resolution, whole-brain T1 -weighted MR images on a 3T Siemens Trio scanner (repetition time = 1620 ms, echo time = 3.87 ms, field of view = 192 × 256 mm, 1 × 1 × 1 mm voxels) using a Siemens 8-channel head coil. Three of 29 patients were contraindicated for a 3T environment, so we collected whole-brain T1 -weighted MR images on a 1.5T Siemens Sonata scanner (repetition time = 3000 ms, echo time = 3.54 ms, field of view = 24 cm, 1.25 × 1.25 × 1.2 mm voxels) using a Siemens 8-channel head coil. Seven of 29 patients were contraindicated for MRI and underwent whole-brain CT scans without contrast (60 axial slices, 3-5 mm slice thickness) on a 64-slice Siemens SOMATOM Sensation scanner. Two patients declined to receive research-quality scans, so we acquired recent clinical MRI (n = 1) and CT (n = 1) scans.
2.3.2 Lesion segmentation and warping to template
For patients with high-resolution MRI scans (n = 19), lesions were segmented manually by trained research assistants on the patients' T1-weighted structural images. Structural images were then registered to a standard template using a symmetric diffeomorphic registration algorithm (Avants, Schoenemann, & Gee, 2006, http://www.picsl.upenn.edu/ANTS). This mapping was used to transform the manually-drawn lesion to the standardized space. To optimize this automated registration process, volumes were first registered to an intermediate template constructed from images acquired on the same scanner. Then, a single mapping from this intermediate template to the Montreal Neurological Institute space “Colin27” volume (Holmes et al., 1998) was used to complete the transformation into standardized space. Subsequently, each lesion map was binarized so that lesioned voxels had a value of 1, and preserved voxels had a value of 0. After being transformed into MNI space using this process, lesion maps were inspected by an experienced neurologist (Dr. H. Branch Coslett) naïve to the behavioral data.
For the remaining patients (n = 12), H.B.C. drew lesions directly onto the Colin27 image using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) after rotating the pitch of the template to approximate the slice plane of the patient's scan. Lesions in native space were visually inspected and analogue areas marked as lesioned on the template. This method achieved high intra- and inter-rater reliability in a previous study (Schnur et al., 2009). Figure 2 displays all lesions on the MNI single-subject brain.
Figure 2.
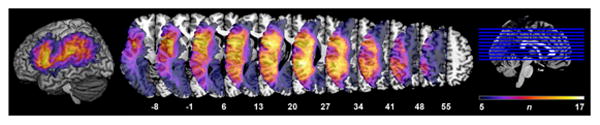
All patient lesions (n = 31) displayed on the Colin27 template in MNI standardized space. Color bar represents the number of patients with lesions at a particular voxel (min = 5; max = 17). Voxels in which fewer than 5 patients had a lesion were not included in VLSM analyses and so are not displayed.
2.3.3 Voxel-based lesion-symptom analyses
VLSM analyses were performed using the VoxBo brain imaging package (Kimberg & Aguirre, 2001). In this method, a t-test is performed at each voxel to compare the behavioral scores of patients with and without lesions. We only performed t-tests for voxels in which at least 5 patients had a lesion; doing so ensured that effects at a given voxel were not driven by a small number of patients. Resulting voxel-wise maps of t-values were corrected for multiple comparisons using a False Discovery Rate (FDR) (Benjamini & Hochberg, 1995) of q = .05, where q is the expected proportion of false positives among supra-threshold voxels (i.e., 5%). Furthermore, we excluded clusters of fewer than 5% of supra-threshold voxels for each analysis; doing so eliminated clusters that could consist entirely of false positive voxels. Statistical maps for display were created with MRIcron; all three-dimensional renderings are shown at a search depth of 8 mm.
VLSM analyses examined lesions that impaired overall gesture pantomime accuracy, or accuracy specifically on the hand action component separately for conflict and non-conflict tools. We also determined the degree to which each patient was disproportionately impaired at producing the hand action for conflict relative to conflict tools using patients' standardized residual scores from regressing conflict tool hand action accuracy on non-conflict tool hand action accuracy (see above).
2.3.4 White matter fiber tract overlap analysis
Finally, following Baldo & colleagues (2012) and Schwartz & colleagues (2012), we characterized the location of white matter voxels that surpassed the VLSM statistical threshold by calculating their overlap with the Johns Hopkins DTI-based probabilistic white matter tractography atlas (Mori et al., 2008), thresholded at 25% probability.
3. Results
3.1 Behavioral Analyses
3.1.1 Tool use pantomime
Descriptive information on overall tool use pantomime and tool use recognition accuracy is presented in Table 2. To determine the effects of use/grasp conflict on pantomime accuracy, we used a three-way mixed ANOVA with conflict (conflict tools, non-conflict tools) and action component (hand action, arm action, amplitude, timing) as within-subjects factors, and participant group (LCVA, control) as a between-subjects factor. We found main effects of conflict [F(1, 45) = 7.10, p = .01], action component [F(2.07, 93.14) = 37.88, p < .0005], and group [F(1, 45) = 32.30, p < .0005], and a significant three-way interaction between these factors [F(2.38, 107.09) = 5.70, p = .003] (Figure 3) (Greenhouse-Geisser correction for violations of sphericity). Tests of simple main effects demonstrated that patients had significantly lower hand action accuracy for conflict versus non-conflict tools (p < .0005), while arm action (p = .61), amplitude (p = .32), and timing (p = .24) accuracy did not differ for patients between the two types of tools. For control participants, accuracy on hand action (p = .41), arm action (p = .85), amplitude (p = .83), and timing (p = 1.0) did not differ significantly between conflict and non-conflict tools. Additionally, patients had significantly lower accuracy than control participants for both types of tools on all four action components (all p < .005). All patterns of significance reported above remained unchanged when we re-ran the analyses excluding the two comparably young patients (31 and 33 years old).
Table 2.
Means and standard deviations for overall accuracy on tool use pantomime and tool use recognition tasks.
| Task | Group | n | Conflict tools | Non-conflict tools | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| M | SD | M | SD | |||
| Tool use pantomime | ||||||
| LCVA | 31 | .78 | .14 | .83 | .10 | |
| Control | 16 | .97 | .03 | .98 | .03 | |
| Tool use recognition | ||||||
| LCVA | 31 | .88 | .12 | .86 | .11 | |
| Control | 14 | .93 | .05 | .95 | .07 | |
Figure 3.
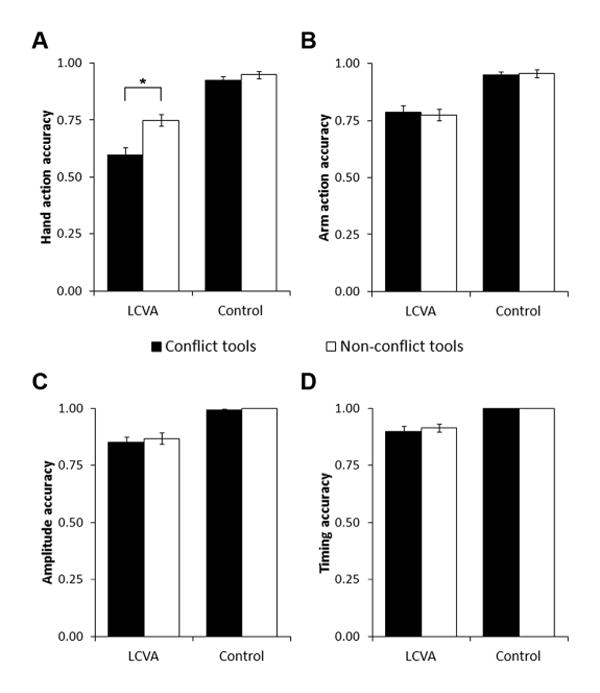
Accuracy for LCVA patients and control participants on each component of tool use pantomime: A) hand action, B) arm action, C) amplitude, and D) timing. Significant pairwise differences between conflict and non-conflict tools are noted with an asterisk. Patients' accuracy was lower than controls' on all components and tool types, so we do not indicate those significant differences here. Error bars represent +/-1 standard error of the mean. *, p < .0005
Some measures of action component accuracy did not pass a test for normality (Shapiro-Wilk Test). Therefore, we repeated the critical pairwise comparisons between conflict and non-conflict accuracy for each component and each group using the non-parametric Wilcoxon Signed-Rank test. As before, LCVA patients continued to show significantly lower hand action accuracy for conflict relative to non-conflict tools (Z = -4.61, p < .0005); there were no significant differences between tool types in arm action (Z = -.35, p = .73), amplitude (Z = -1.08, p = .28), or timing (Z = -1.16, p = .25) accuracy. For control participants, there were no significant differences between conflict and non-conflict tools on hand action (Z = -1.44, p = .15), arm action (Z = -1.16, p = .25) amplitude (Z = -1.34, p = .18), or timing (all values at ceiling for both tool types) accuracy.
For LCVA patients, we also investigated the relationship between use/grasp conflict and the severity of apraxia as defined by performance on the pantomime task. We performed a non-parametric (Spearman) correlation between the standardized residual scores from regressing conflict on non-conflict hand action accuracy (see Section 2) and patients' overall accuracy on the pantomime task across the four action components. This analysis thus assessed whether patients with poorer total pantomime scores (a measure often used in the literature to define apraxia) were disproportionately impaired on the hand action component of conflict as compared to non-conflict tools. There was indeed a reliable correlation between overall apraxia severity and the magnitude of the conflict effect in the hand action component [rho(29) = .36, p = .04].
3.1.2 Hand action errors
We used a two-way repeated measures ANOVA to examine the effects of conflict on the percentage of hand action errors of each type (prehensile, non-prehensile, hybrid, BPO, multiple attempts, same as use, other) produced by LCVA patients (Figure 4). There was a significant main effect of conflict [F(1, 30) = 34.81, p < .0005], a main effect of error type [F(3.22, 96.46) = 22.60, p < .0005] and a significant conflict × error type interaction [F(3.68, 110.43) = 6.34, p < .0005] (Green house-Geisser correction for violations of sphericity). Tests of simple main effects showed that patients made more prehensile (p = .02) and multiple attempt (p < .0005) errors and tended to make more hybrid errors (p = .09) on conflict versus non-conflict tools. There were no differences between the numbers of non-prehensile (p = .52), BPO (p = .57), same as use (p = .68), or other (p = .15) errors between the two tool types.
Figure 4.
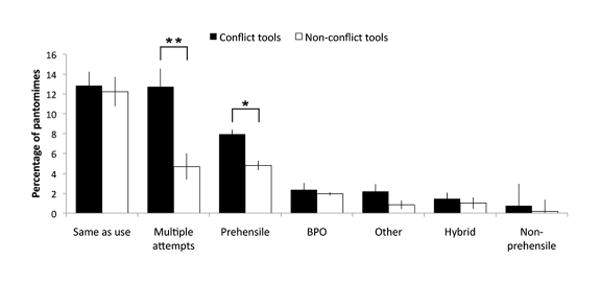
Different types of hand action errors made by patients to conflict and non-conflict tools in the pantomime task, expressed as a percentage of all trials of each type. BPO = body-part-as-object. Error bars represent +/-1 standard error of the mean. *, p < .05; **, p < .0005
Given violations of normality among the distributions of error types, we repeated the comparisons between conflict and non-conflict for each type of error using a non-parametric test (Wilcoxon Signed-Rank test). Patients continued to make significantly more multiple attempt (Z = -4.28, p < .005) and prehensile (Z = -2.50, p = .01) errors, and tended to make more hybrid (Z = -1.80, p = .07) and other (Z = -1.82, p = .07) errors, to conflict versus non-conflict tools. No significant effects of conflict were found on non-prehensile (Z = -.11, p = .92), BPO (Z = -1.40, p = .16), or same as use (Z = -.55, p = .58) errors.
3.1.3 Action recognition
To determine whether use/grasp conflict affected the selection of actions for output rather than a putatively earlier stage of processing that might impact both recognition and production, we looked for effects of conflict and participant group on the recognition of tool use actions using a two-way mixed ANOVA. Although patients performed less accurately than control participants overall [F(1, 43) = 6.94, p = .01], there was no effect of use/grasp conflict on recognition accuracy [F(1, 43) = .002, p = .97], nor any interaction between conflict and participant group [F(1, 43) = 1.65, p = .21] (Table 2). Thus, patients were not more likely than controls to exhibit sensitivity to conflict in the action recognition task. This pattern of results held using a non-parametric test (Wilcoxon Signed-Rank test): neither LCVA patients (Z = -1.35, p = .18) nor control participants (Z = -1.11, p = .27) exhibited differences between recognition of conflict versus non-conflict tool actions. This result indicates that use/grasp conflict arose only when patients had to select an action for execution, not during recognition of tools or tool actions.
3.1.4 Subset analysis
To ensure that the observed differences between conflict and non-conflict tools could not be attributed to insufficient matching of affordance strength, name agreement, or familiarity, or to differences in the number of moveable parts between the tool types, we repeated our analyses of tool use pantomime accuracy using a better matched subset of tools (n = 10 conflict tools, n = 10 non-conflict tools; see Section 2.2.1.2).
First, we examined accuracy on this subset of tools using a three-way mixed ANOVA. We found significant main effects of conflict [F(1, 45) = 9.34, p = .004], action component [F(2.208, 99.366) = 27.71, p < .005], group [F(1, 45) = 30.50, p < .005], and a marginally significant three-way interaction [F(2.206, 99.261) = 2.84, p = .058] (Greenhouse-Geisser correction for violations of sphericity). Given our hypotheses regarding the specificity of conflict effects to the hand action accuracy of LCVA patients, we explore this interaction further. Tests of simple main effects demonstrated that patients had significantly lower hand action accuracy for conflict versus non-conflict tools (p < .0005), while arm action (p = .31), amplitude (p = .53), and timing (p = .19) accuracy did not differ for patients between the two types of tools. For control participants, accuracy on hand action (p = .14), arm action (p = .55), amplitude (p = .73), and timing (p = 1.0) did not differ significantly between conflict and non-conflict tools. Additionally, patients had significantly lower accuracy than control participants for both types of tools on all four components (all p < .01).
We also performed non-parametric tests (Wilcoxon Signed-Rank test) on accuracy to this subset of tools. When pantomiming tool use, LCVA patients had significantly lower hand action accuracy (Z = -4.2, p < .005), but not arm action (Z = -.86, p = .39), amplitude (Z = -.44, p = .66), or timing (Z = -.83, p = .41) accuracy, on conflict versus non-conflict tools. While control participants continued to show no significant differences between conflict and non-conflict tools on arm action (Z = -1.15, p = .25), amplitude (Z = -1.41, p = .16), or timing (all values at ceiling for both tool types) accuracy, they exhibited significantly lower hand action accuracy on conflict tools (Z = -2.41, p = .02). Note that this result is more modest than that seen with the patients (cf. Z = -4.2, p < .005). Nevertheless, it is consistent with those of Jax & Buxbaum (2010), who found that neurologically-intact participants were slower to initiate use actions to conflict versus non-conflict tools. Together, the results of this subset analysis indicate that even when conflict and non-conflict tools are well-matched for familiarity, affordance strength, name agreement, and number of moveable parts, differences between the hand action accuracies of the two tool types persist.
3.2 VLSM Analyses
To situate our findings within the context of tool use, more broadly, we first identified brain regions critical for pantomiming tool use (i.e., overall pantomime accuracy) (Table 3, Figure 5A). Consistent with prior findings in the literature (Buxbaum et al., 2007, 2014; Manuel et al., 2013), lesions to a broad network of areas, including IPL, posterior temporal cortex (pTC), pre- and post-central gyri, and inferior prefrontal cortex, were associated with impaired pantomime accuracy (FDR q = .05).
Table 3. Suprathreshold clusters from VLSM analyses.
| Number of voxels | x | y | z | Location of peak value within cluster |
|---|---|---|---|---|
| Overall tool use pantomime accuracy (FDR q = .05) | ||||
| 81981 | -24 | -24 | 12 | White matter (internal capsule) |
| Residualized hand action scores for conflict tools (FDR q = .05) | ||||
| 16577 | -65 | -29 | 36 | Left supramarginal gyrus |
| 3358 | -33 | 9 | -12 | Left anterior insula |
| Residualized hand action scores for conflict tools, subset analysis (FDR q = .05) | ||||
| 2259 | -64 | -32 | 31 | Left supramarginal gyrus |
| Conjunction of hand action accuracy for conflict and non-conflict tools (FDR q = .06) | ||||
| 1758 | -50 | -40 | 1 | Left posterior middle temporal gyrus |
| 1057 | -24 | -31 | 48 | White matter (centrum semiovale) |
| 363 | -35 | -42 | 55 | Left anterior intraparietal sulcus |
| 277 | -32 | -62 | 28 | White matter (corona radiata/centrum semiovale) |
| 222 | -26 | -26 | 15 | White matter (external capsule) |
Note: Coordinates represent the location of the peak value within a cluster in MNI standardized space. Only clusters containing at least 5% (or 6%, for the conjunction) of supra-threshold voxels are reported in the table.
Figure 5.
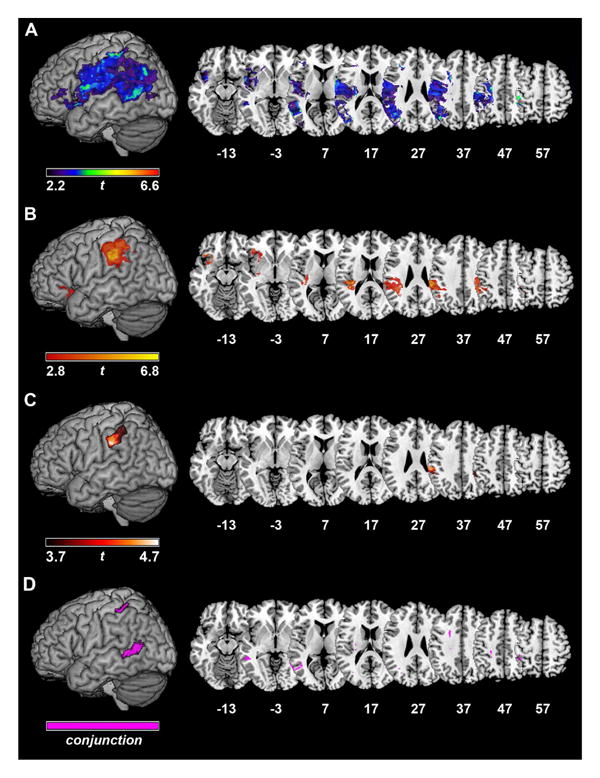
VLSM analyses for A) overall tool use pantomime accuracy (FDR q = .05), B) residualized hand action scores for conflict tools (FDR q = .05), C) residualized hand action scores for a subset of conflict tools (FDR q = .05), and D) the conjunction of individual VLSM maps for conflict and non-conflict tool hand action accuracy (FDR q = .06).
Next, we sought to determine the neural basis of the conflict effect described earlier: that is, disproportionately poor hand action accuracy for conflict tools relative to non-conflict tools. The majority of significant voxels (69.74%) associated with poorer hand action accuracy for conflict tools (i.e., lower residualized hand action scores) were located in grey matter, determined by calculating the overlap between supra-threshold voxels and the entirety of the Automated Anatomical Labeling Atlas of grey matter (Tzourio-Mazoyer et al., 2002). A large cluster of voxels was located in the SMG (Table 3, Figure 5B) (FDR q = .05). A smaller cluster of voxels within the anterior insula and inferior frontal gyrus (IFG) also reached significance.
Finally, we repeated this analysis using residualized hand action scores derived from the subset of items (n = 10 conflict tools; n = 10 non-conflict tools) more closely matched for number of moveable parts, familiarity, strength of affordance, and name agreement (see Section 2.2.1.2). We found a single supra-threshold cluster in SMG associated with poorer hand action accuracy for conflict tools (FDR q = .05), the majority of which (98.14%) was located in grey matter (Table 3, Figure 5C). Unlike the analysis using all tools, the subset analysis did not reveal any significant voxels in the anterior insula or IFG at a threshold of q = .05; however, these regions emerged at a more lenient threshold (uncorrected p = .01), indicating that the pattern of results is similar.
3.3 Exploratory Conjunction Analyses
The previous analyses uncovered areas associated with differentially poor hand action accuracy for conflict tools. Lesions to these areas may specifically impair performance on conflict tools, or they may also impair performance on non-conflict tools, just to a lesser extent. To adjudicate between these possibilities, we first determined the lesion sites associated with impaired hand action accuracy separately for conflict and non-conflict tools; that is, we performed two VLSM analyses, one using raw hand action accuracy for conflict tools, and the other using raw hand action accuracy for non-conflict tools. By examining the supra-threshold voxels shared by these two analyses, we can determine whether damage to a brain region yields significant hand action impairments for all tools rather than just conflict tools. We chose to examine the conjunction between hand action accuracy separately for conflict and non-conflict tools rather than the average accuracy of these two conditions since the VLSM results for an average score could be skewed by a strong association between a brain region and one tool type but not the other. A conjunction analysis instead reveals brain areas that significantly contribute to both conditions.
For non-conflict tools, only two small clusters (22 and 11 voxels, respectively), located entirely within white matter, survived a threshold of FDR q = .05 and a 5% of supra-threshold voxels cluster-size threshold (see Materials & Methods). This paucity of significant voxels may be a result of the higher hand action accuracy (M = 75%) and lower variability (SD = .14) for non-conflict tools compared to conflict tools (M = 60%, SD = .20). Therefore, we lowered the threshold very slightly to FDR q = .06 (i.e., 6%, rather than 5%, of supra-threshold voxels may be false positives) for both VLSM analyses (hand action accuracy of conflict and non-conflict tools). We then computed the conjunction of these two thresholded statistical maps (Nichols, Brett, Andersson, Wager, & Poline, 2005) (Table 3, Figure 5D). Lesions to posterior middle temporal gyrus (pMTG), anterior intraparietal sulcus (aIPS), and several white matter fiber tracts (internal capsule, external capsule, and centrum semiovale) were associated with lower hand action accuracy for both conflict and non-conflict tools. Thus, importantly, brain regions responsible for enabling selection between use and grasp actions (SMG, anterior insula/IFG), described earlier, are different from the regions that subserve overall hand action accuracy.
3.4 Exploratory Lesion Subtraction Analysis
As noted earlier, in patients' behavior, we found a significant but relatively modest relationship between the size of the conflict effect and apraxia severity. Limitations in the strength of this association might reflect the non-uniformity of lesions causing apraxia; for example, there may be a neuroanatomically-defined subtype of apraxia with SMG lesions in which conflict effects are relatively prominent, whereas conflict effects may be less clear in apraxics with lesions outside of SMG. Such a finding would be of considerable interest given long-standing confusion about the relationship of various types of apraxia and their neuroanatomic substrates (see, e.g., Buxbaum, 2001). To test this “subtype” hypothesis, we used the lesion subtraction method (e.g., Goldenberg & Karnath, 2006). First, we performed a median split of overall pantomime accuracy to determine which patients exhibited more severe apraxia, and a median split of standardized hand action residual scores to determine which patients exhibited larger or smaller effects of use/grasp conflict. We then selected the apraxic patients with larger conflict effects (n = 9) and the apraxic patients with smaller/no conflict effects (n = 6) and calculated the percentage of lesion overlap within each of these two groups. Finally, we subtracted these two percentage maps from each other to find voxels associated with at least a 56% difference between groups (Figure 6A); this difference corresponds to a chi-squared test between groups significant at the .05 level (see Kemmerer, Rudrauf, Manzel, & Tranel, 2012; Mirman & Graziano, 2013 for more details). We found that apraxic patients who exhibited larger effects of use/grasp conflict tended to share lesions in left SMG, while apraxic patients who exhibited smaller effects of conflict tended to share lesions in left posterior temporo-occipital cortex (lateral occipital cortex and posterior middle temporal gyrus). Critically, a Mann Whitney test indicated that the lesion volumes of patients with larger conflict effects (M = 94390, SD = 24155) did not differ from patients with smaller conflict effects (M = 130491, SD = 30330) (U = 18.0, p = .29). These results lend support to the possibility that while lesions to multiple brain areas can produce apraxia (see Buxbaum et al., 2014), lesions to left SMG result in a specific type of apraxia in which patients have more difficulty selecting among different possible tool actions. We will expand upon this interpretation in the Discussion.
Figure 6.
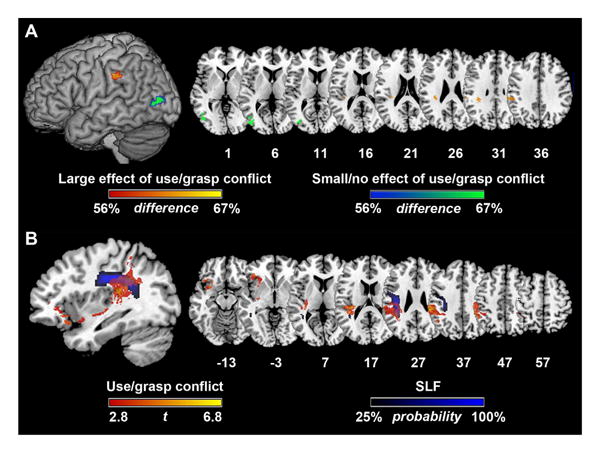
A) Among patients with moderate-to-severe apraxia, difference between the number of patients with larger (n = 9; red/orange) or smaller (n = 6; blue/green) effects of use/grasp conflict on pantomime. Overlap maps thresholded at 56% (e.g., at each voxel, at least a 56% difference between the number of apraxic patients with larger versus smaller conflict effects). This threshold is equivalent to a chi-squared test between groups significant at the .05 level. B) Probabilistic location of the superior longitudinal fasciculus (SLF) from the JHU white matter atlas (black/blue) overlaid onto VLSM results for use/grasp conflict (residualized hand action scores for conflict tools; FDR q = .05). Probabilistic SLF map thresholded at 25%.
3.5 White Matter Fiber Tract Overlap Analysis
Finally, we examined the extent to which voxels associated with disproportionately worse hand action accuracy for conflict tools (Figure 5B) overlapped with known white matter fiber tracts. Supra-threshold voxels in this analysis overlapped with 19.61% of voxels in the left superior longitudinal fasciculus (SLF) (Figure 6B), a fiber pathway that connects temporoparietal regions with the frontal lobe (Makris et al., 2005). Overlap with other white matter tracts was minimal (5% or less).
4. Discussion
Although tools evoke their actions even when task-irrelevant (e.g., Jax & Buxbaum, 2010), the mechanisms by which appropriate tool actions are selected—and their neural correlates—are poorly understood. Here, we assessed the performance of LCVA patients on a production task in which the grasp-to-move and use actions for a tool were congruent or incongruent. We also used VLSM and tractographic overlap analyses to determine brain regions necessary for selecting among tool-directed actions. Finally, we examined patients' behavior and lesions to assess whether an inability to select between different tool-directed actions constitutes a sub-type of apraxia.
When pantomiming the use of familiar tools, LCVA patients' ability to produce the correct hand action (but not arm action, amplitude, or timing)3 was significantly affected by conflicting use and grasp actions. Control participants showed no effects of conflict when we examined performance on all tools, but significantly lower hand action accuracy for a subset of well-matched conflict versus non-conflict tools using a non-parametric comparison. These findings replicate and extend those of Jax & Buxbaum (2013), from an arguably artificial task (positioning the hand on a tool as if to use it) to a task often used to diagnose apraxia (pantomiming tool use). This is the first study to demonstrate a reliable link between a characteristic of tools (that is, whether they are associated with conflicting use and grasp actions) and errors on a typical praxis task. Furthermore, neither LCVA patients nor control participants were significantly affected by use/grasp conflict when recognizing tool use pantomimes. Thus, the fundamental bottleneck arises from the need to select a single action for production consistent with task goals (here, demonstrating tool use), not when merely accessing the meanings of actions. We account for these data below by considering how information from vision and semantic memory are reconciled during the selection of tool actions.
First, we note that the problem of selecting between different use and grasp actions associated with the same tool is a specific—albeit frequently encountered—instance of the action selection process, more generally. Early accounts of action selection assumed that deciding “what to do” (selection) occurred before determining “how to do it” (preparation and execution) (e.g., Keele, 1968; Newell & Simon, 1972). However, mounting evidence from neurophysiology, neuroimaging, and human behavior indicates that these processes are intimately intertwined and unfold in parallel (e.g., Cisek, 2007; Cisek & Kalaska, 2005; Kim & Shadlen, 1999; Spivey, 2007; Tipper, Howard, & Houghton, 2000; see Cisek & Kalaska, 2010 for a review), likely as a biased competition between different possible actions (Cisek, 2007; Pastor-Bernier & Cisek, 2011). Critically, one characteristic of such a mechanism is that inappropriate actions remain active throughout the competitive process and thus have the potential to influence ongoing behavior (e.g., Spivey & Dale, 2006). Yet, as noted by Filevich & Haggard (2013, p. 1), unselected actions “…have proved difficult to study for the simple methodological reason that they have no behavioral output”. In this vein, the overt errors made by patients with apraxia offer an opportunity to study the influence of automatically-evoked but inappropriate actions on the action selection process, and the brain regions required for successful selection.
VLSM analyses revealed that lesions to left SMG and IFG/anterior insula yielded disproportionately poor hand action accuracy for conflict versus non-conflict tools. On the other hand, lesions to pMTG and aIPS tended to be associated (at q = .06) with lower hand action accuracy for both kinds of tools. Lesion subtraction analyses supported this distinction between regions: apraxic patients with larger effects of use/grasp conflict shared lesions in SMG, while apraxic patients with smaller effects of conflict shared lesions in pTC. These findings add specificity to our understanding of the brain regions that support tool use and suggest that a specific SMG-involved subtype of apraxia may be characterized by a heightened sensitivity to the presence of conflicting use/grasp actions.
Studies of anatomical connectivity find that left hemisphere regions involved in tool use are strongly anatomically interconnected: specifically, SMG is connected to pMTG, vPMC/IFG, somatosensory, and superior and intraparietal areas (Caspers et al., 2011; Ramayya, Glasser, & Rilling, 2010) via the SLF (Makris et al., 2005). Our results shed light on the different functional roles for these interconnected nodes of the tool use network. First, left SMG appears to play a critical role in enabling selection of appropriate tool-directed hand actions given conflicting alternatives. On some accounts, SMG stores learned representations of hand and limb postures for functionally interacting with tools (Pelgrims, Olivier, & Andres, 2011; Vingerhoets, 2008). However, evidence outside the tool domain suggests a broader role for SMG in action, for instance, greater activation within left IPL/SMG during the simultaneous activation of incompatible responses during the Eriksen flanker and other similar tasks (Hazeltine et al., 2000; Schumacher, Elston, & D'Esposito, 2003).
Based on SMG's strongly left-lateralized connectivity with pMTG (a critical substrate of action semantics, e.g., Kalenine, Buxbaum, & Coslett, 2010), Ramayya & colleagues (2010) suggested that SMG integrates spatial with non-spatial/semantic information to generate an action plan (see also Randerath et al., 2010). This account is consistent with the present data and with our prior claims regarding the role of IPL in integrating information from dorsal and ventral visual processing streams (Buxbaum, Johnson-Frey, et al., 2005; Buxbaum et al., 2007, 2003). Following a similar proposal from Cisek & colleagues (Cisek, 2007; Cisek & Kalaska, 2010), candidate grasp and use actions from the dorsal (aIPS) and ventral streams (pMTG) (see Binkofski & Buxbaum, 2013; for details about this neuroanatomical division of labor Buxbaum & Kalenine, 2010) may be represented simultaneously within SMG. Further, SMG may receive from PFC/IFG a biasing signal regarding task context and goals (Miller & Cohen, 2001; Thompson-Schill & Botvinick, 2006). This bias boosts one representation in SMG closer to the threshold for production (or inhibits the inappropriate representation). Given connectivity between IFG and SMG, and the present finding that lesions to the white matter pathway that connects these two regions (SLF) are associated with use/grasp conflict effects, we suggest that damage to the IFG/SLF/SMG pathway disrupts the mechanisms by which this biased competition normally enables selection of appropriate tool actions. Consistent with this claim, Randerath & colleagues (2010) found that lesions to IFG impair the selection of grasps for subsequent tool use but not subsequent tool transport, and they propose that this region uses context and task goals to select appropriate actions. In the current study, analysis of patients' hand action errors is also consistent with this view. When demonstrating the use of conflict tools, LCVA patients produced disproportionately more prehensile actions and multiple attempts at the target action, a result that suggests selection of a task-inappropriate alternative specified by the dorsal stream (i.e., grasping) or an inability to select a single response.
While SMG and IFG are critical for resolving action competition, lesions to pMTG and aIPS impaired hand action accuracy for all tools, irrespective of use/grasp conflict. We suggest that these regions provide input to the competitive process. pMTG is active when participants view or make judgments about tools and their actions(e.g., Kellenbach, Brett, & Patterson, 2003), and lesions here impair recognition of actions (Kalenine et al., 2010). Yet, this region also participates in generating tool actions. For instance, patterns of activity within pMTG discriminate between different prepared tool actions (Gallivan, McLean, Valyear, & Culham, 2013). Additionally, a recent VLSM study from our lab examined tool-related and imitative actions in sample of 71 LCVA patients (5 of whom also participated in the current study) (Buxbaum et al., 2014). We found that pMTG lesions impair hand/arm positioning both when patients imitate tool use actions performed by an experimenter and when patients pantomime tool use actions in response to viewed tools. pMTG lies anterior to areas specialized for processing visual motion (area hMT+, J. D. G. Watson et al., 1993) and is particularly responsive to the rigid, unarticulated motion characteristic of tools (Beauchamp & Martin, 2007). Thus, this region may represent knowledge of actions derived from visual experience (Kalenine et al., 2010; Orban & Caruana, 2014; C. E. Watson, Cardillo, Ianni, & Chatterjee, 2013), including the typical posture and movement of the hand (Buxbaum, 2001; Buxbaum et al., 2014; C. E. Watson & Buxbaum, 2014). During action, pMTG may provide SMG with information about the visuomotor characteristics of the target use action. Without this critical input, performance is impaired for any tool for which an appropriate use action must be generated, i.e., for both conflict and non-conflict tools.
Lesions to aIPS were also associated with impaired hand action accuracy for all tools. Generally, lesions to aIPS impair object grasping (Binkofski et al., 1998), and aIPS is active when participants make simple grasping movements (e.g., Binkofski et al., 1998; Culham et al., 2003) or observe graspable objects (Grèzes & Decety, 2002). This evidence is consistent with the claim that aIPS extracts information relevant for shaping the hand based on an object's physical structure (Oztop & Arbib, 2002; Sakata et al., 1998). In the current study, lesions to aIPS may have disrupted performance even for tools used with non-prehensile hand postures (e.g., keyboard) by preventing patients from tailoring use actions on-line to the physical structure of the currently perceived tool (e.g., a tool's unique shape, size, or orientation) (but see Hamilton & Grafton, 2006; Tunik, Rice, Hamilton, & Grafton, 2007 for alternative accounts focusing on action goals). Information from aIPS may influence competition within SMG via direct anatomical connections (Caspers et al., 2011), or these two regions may send converging signals to vPMC in parallel (Orban & Caruana, 2014).
Notably, a recent fMRI study by Schubotz, Wurm, Wittman, & von Cramon (2014) is consistent with the account we propose. Participants viewed correctly or incorrectly performed actions with objects (tools) associated with varying numbers of actions. Activation in left aIPS—not encroaching on SMG—and left pMTG increased with the number of actions associated with objects, irrespective of the correctness of the action. On the other hand, activation within these same areas bilaterally, with the addition of bilateral anterior SMG, premotor cortex, and mid-insula, increased with the number of actions to a greater degree during observation of correct versus incorrect actions. This pattern of results is consistent with the present suggestion that pMTG and aIPS represent the automatically-evoked tool use and grasp actions that constitute input for the competitive action selection process. By contrast, Schubotz & colleagues propose that activation in SMG, premotor cortex, and insula “… increase[s] with the competition load between object-evoked action options” (2014, p. 10).
In the current study, we manipulated the level of use/grasp conflict present in a set of tools while keeping conflict and non-conflict tools matched for other tool properties—familiarity, affordance strength, name agreement, and, in a subset analysis, number of moveable parts. However, these factors may also influence the ease with which patients can demonstrate tool use. For example, patients with apraxia may more easily retrieve use actions for very familiar tools, in much the same way that patients with aphasia have less difficulty naming familiar objects (e.g., Hirsh & Funnell, 1995; Nickels & Howard, 1995). Similarly, it may be more difficult to demonstrate the use of tools that have more moveable parts and are thus more mechanically complex. In fact, deficient mechanical problem solving (Goldenberg & Hagmann, 1998; Goldenberg & Spatt, 2009) or technical reasoning (Jarry et al., 2013; Osiurak et al., 2009) have been suggested as accounts of apraxic errors and the effects of parietal lobe lesions. In the current study, we performed post-hoc VLSM (and behavioral) analyses on conflict and non-conflict tools matched for number of moveable parts; in this analysis, lesions to SMG (and, at a more lenient threshold, IFG/anterior insula) remained predictive of disproportionately poor hand action accuracy to conflict tools. This result indicates that the effect of conflicting use and grasp actions on apraxic errors cannot be reduced to mechanical complexity. One difficulty with interpreting these results, however, is that moving parts that influence mechanical complexity, even if not associated with typical use or grasp actions, may nevertheless “afford” additional novel actions that may compete for selection (albeit perhaps more weakly). In other words, the “selection difficulty” and “mechanical reasoning” accounts do not make mutually exclusive predictions. Future studies with carefully designed stimuli may help to disambiguate these possibilities.
In the current study, we investigated the effects of use/grasp conflict on tool use pantomime, but the implications of our results for action competition during actual tool use remain an open question. To date, no research has addressed the role of competition on tool use. Yet, several pieces of data suggest that the effects of use/grasp conflict may extend to actual tool use. First, healthy participants in Jax & Buxbaum (2010) did not pantomime tool use actions. Instead, they reached out and positioned their hands on tools as if to actually use them, and these participants were nevertheless affected by use/grasp conflict. Second, as noted earlier, although apraxic performance improves with real tools (Poizner et al., 1990), LCVA patients' accuracy on tool use pantomime is significantly correlated with actual tool use (Jarry et al., 2013), as are the characteristics of their movement errors in these two tasks (Hermsdörfer et al., 2013). Finally, pantomime accuracy is predictive of other apraxic behaviors, like errors during mealtime eating (Foundas et al., 1995) and increased reliance on caregiver assistance (Hanna-Pladdy, Heilman, & Foundas, 2003). For these reasons, we expect that apraxic patients will have more difficulty actually using tools with conflicting use and grasp hand actions, as is currently being assessed in a study underway in our laboratory.
Finally, we note that, to our knowledge, this is the first study to investigate apraxic error types as a function of tool characteristics. By contrast, the study of errors is a fruitful area of inquiry within the language domain. In particular, patients with conduction aphasia make errors that bear striking resemblance to the multiple attempt errors observed in the current study: during “conduite d'approche”, a patient makes repeated, unsuccessful attempts at producing a target phoneme (Goodglass, 1992). Conduction aphasia is often associated with lesions to a portion of the SLF medial to the SMG and upper insula (arcuate fasciculus; see Bernal & Ardila, 2009 for a review). In the current study, a substantial portion of the SLF (defined with JHU white matter atlas, Mori et al., 2008) overlapped with voxels associated with disproportionate conflict tool impairments. Although speculative, the similarity between error types and lesion location in the current study and those in conduction aphasia hints that both disorders may reflect an inability to resolve competition between actions—be they skilled tool use actions or spoken language. More research is required to determine whether these similarities reflect damage to domain-general mechanisms that broadly resolve action competition or to domain-specific mechanisms located adjacent to each other within the IFG/SLF/SMG pathway.
5. Conclusions
We investigated the mechanisms and corresponding brain regions necessary for selecting between different “use” and “grasp-to-move” actions associated with the same tool. Our results revealed that while lesions to pMTG and aIPS impaired production of use actions for all tools, lesions to SMG, IFG/anterior insula, and the superior longitudinal fasciculus specifically impaired production of use actions for tools used and grasped with different hand actions. Furthermore, the nature of patients' errors to “conflict” tools was consistent with inappropriate selection of grasping actions or difficulty selecting single actions. By contrast, use/grasp conflict did not significantly affect action recognition. We propose that this pattern of data reflects a SMG/SLF/IFG pathway that implements biased competition between possible tool actions, with pMTG/aIPS providing necessary input to the competitive process. Furthermore, our results reveal that studying the parallel activation of and competition between representations can be informative with respect to the organization of the tool action system. As in other domains (e.g., semantic memory, Schnur, Schwartz, Brecher, & Hodgson, 2006; decision making, McKinstry, Dale, & Spivey, 2008; voluntary action, Filevich & Haggard, 2013), behavioral paradigms that encourage competition can be similarly used to understand the mechanisms that support successful tool use.
Supplementary Material
Figure S1. Conflict and non-conflict tool photographs used as experimental stimuli (photographs from the BOSS database, Brodeur et al., 2010). Tools included in the highly-matched subset are marked with an asterisk.
Acknowledgments
This research was funded by National Institutes of Health (NIH) grants R01NS065049 (LJB) and T32HD007425 (CEW, trainee). We thank Allison Shapiro and Alexis Kington for their help in stimulus development and data collection, H. Branch Coslett for his help with lesion segmentation, and Allison Shapiro, Alexis Kington, and Leyla Tarhan for coding patients' gestures.
Footnotes
Overall tool use pantomime accuracy of the new control participants (M = 96.5, SD = .04) did not differ from that of re-tested control participants (M = 97.5, SD = .01) [t(5.84) = -.62, p = .56].
We chose this wording to avoid participants tallying parts of an object that move incidentally when the object is moved, such as decorative cords on a bongo drum.
We note that it may be more difficult to detect errors of amplitude or timing, relative to errors of hand or arm action, with the action coding method we employ here. Indeed, kinematic analyses may more sensitively detect spatiotemporal errors in patients with apraxia (Haaland, Harrington, & Knight, 1999; Hermsdörfer et al., 1996). Therefore, we cannot exclude the possibility that such an approach could reveal more subtle effects of use/grasp conflict on amplitude and timing than detected here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10(3):397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26(3-4):338–354. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: Evidence from fMRI studies of tools. Cortex. 2007;43(3):461–468. doi: 10.1016/s0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B. Methodological. 1995;57(1):289–300. [Google Scholar]
- Bernal B, Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain. 2009;132(9):2309–2316. doi: 10.1093/brain/awp206. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buxbaum LJ. Two action systems in the human brain. Brain and Language. 2013;127(2):222–229. doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension A combined lesion and functional MRI activation study. Neurology. 1998;50(5):1253–1259. doi: 10.1212/WNL.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Brodeur MB, Dionne-Dostie E, Montreuil T, Lepage M. The Bank of Standardized Stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PLoS ONE. 2010;5(5):e10773. doi: 10.1371/journal.pone.0010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: A call to action. Neurocase. 2001;7:44–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Giovannetti T, Libon D. The role of the dynamic body schema in praxis: Evidence from Primary Progressive Apraxia. Brain and Cognition. 2000;44(2):166–191. doi: 10.1006/brcg.2000.1227. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43(6):917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kalenine S. Action knowledge, visuomotor activation, and embodiment in the two action systems. Annals of the New York Academy of Sciences. 2010;1191(1):201–218. doi: 10.1111/j.1749-6632.2010.05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–423. doi: 10.1016/S0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research. 2005;25(1):226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JA. Neural substrates of knowledge of hand postures for object grasping and functional object use: Evidence from fMRI. Brain Research. 2006;1117(1):175–185. doi: 10.1016/j.brainres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Shapiro AD, Coslett HB. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain. 2014 doi: 10.1093/brain/awu111. awu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky RL. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41(8):1091–1113. doi: 10.1016/S0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, von Kapri A, Kuhlen T, Huang R, Zilles K. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. NeuroImage. 2011;58(2):362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: The affordance competition hypothesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1485):1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron. 2005;45(5):801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience. 2010;33(1):269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Creem-Regehr SH, Dilda V, Vicchrilli AE, Federer F, Lee JN. The influence of complex action knowledge on representations of novel graspable objects: Evidence from functional magnetic resonance imaging. Journal of the International Neuropsychological Society. 2007;13(06):1009–1020. doi: 10.1017/S1355617707071093. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, De Souza JFX, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Experimental Brain Research. 2003;153(2):180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Filevich E, Haggard P. Persistence of internal representations of alternative voluntary actions. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Macauley BL, Raymer AM, Maher LM, Heilman KM, Gonzalez Rothi LJ. Ecological implications of limb apraxia: Evidence from mealtime behavior. Journal of the International Neuropsychological Society. 1995;1(01):62–66. doi: 10.1017/S1355617700000114. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Hallett M. The role of the dorsal stream for gesture production. NeuroImage. 2006;23(2):417–428. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, Culham JC. Decoding the neural mechanisms of human tool use. eLife. 2013;2(0):e00425–e00425. doi: 10.7554/eLife.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Hagmann S. Tool use and mechanical problem solving in apraxia. Neuropsychologia. 1998;36(7):581–589. doi: 10.1016/S0028-3932(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Karnath HO. The neural basis of imitation is body-part specific. The Journal of Neuroscience. 2006;26(23):6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Spatt J. The neural basis of tool use. Brain. 2009;132(6):1645–1655. doi: 10.1093/brain/awp080. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The Neural Basis of Decision Making. Annual Review of Neuroscience. 2007;30(1):535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature. 1991;343(6305):154–156. doi: 10.1038/349154a0. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Diagnosis of conduction aphasia. In: Kohn SE, editor. Conduction aphasia. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 39–49. [Google Scholar]
- Grèzes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40(2):212–222. doi: 10.1016/S0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight R. Spatial deficits in ideomotor limb apraxia: A kinematic analysis of aiming movements. Brain. 1999;122(6):1169–1182. doi: 10.1093/brain/122.6.1169. [DOI] [PubMed] [Google Scholar]
- Hamilton AFC, Grafton ST. Goal representation in human anterior intraparietal sulcus. The Journal of Neuroscience. 2006;26(4):1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna–Pladdy B, Daniels SK, Fieselman MA, Thompson K, Vasterling JJ, Heilman KM, Foundas AL. Praxis lateralization: errors in right and left hemisphere stroke. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2001;37(2):219–230. doi: 10.1016/s0010-9452(08)70569-0. [DOI] [PubMed] [Google Scholar]
- Hanna–Pladdy B, Heilman KM, Foundas AL. Ecological implications of ideomotor apraxia Evidence from physical activities of daily living. Neurology. 2003;60(3):487–490. doi: 10.1212/WNL.60.3.487. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Bestmann S, Ward NS, Woerbel S, Mastroeni C, Granert O, Siebner HR. Left dorsal premotor cortex and supramarginal gyrus complement each other during rapid action reprogramming. Journal of Neuroscience. 2012;32(46):16162–16171. doi: 10.1523/JNEUROSCI.1010-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JDE. Neural activation during response competition. Journal of Cognitive Neuroscience. 2000;12(supplement 2):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Li Y, Randerath J, Roby-Brami A, Goldenberg G. Tool use kinematics across different modes of execution. Implications for action representation and apraxia. Cortex. 2013;43(1):184–199. doi: 10.1016/j.cortex.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Mai N, Spatt J, Marquardt C, Veltkamp R, Goldenberg G. Kinematic analysis of movement imitation in apraxia. Brain. 1996;119(5):1575–1586. doi: 10.1093/brain/119.5.1575. [DOI] [PubMed] [Google Scholar]
- Hirsh KW, Funnell E. Those old, familiar things: Age of acquisition, familiarity and lexical access in progressive aphasia. Journal of Neurolinguistics. 1995;9(1):23–32. doi: 10.1016/0911-6044(95)00003-8. [DOI] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jarry C, Osiurak F, Delafuys D, Chauviré V, Etcharry-Bouyx F, Le Gall D. Apraxia of tool use: More evidence for the technical reasoning hypothesis. Cortex. 2013;43(9):2322–2333. doi: 10.1016/j.cortex.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ. Response interference between functional and structural actions linked to the same familiar object. Cognition. 2010;115(2):350–355. doi: 10.1016/j.cognition.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ. Response interference between functional and structural object-related actions is increased in patients with ideomotor apraxia. Journal of Neuropsychology. 2013;7(1):12–18. doi: 10.1111/j.1748-6653.2012.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends in Cognitive Sciences. 2004;8(2):71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Perenin MT. Cortical control of visually guided reaching: Evidence from patients with optic ataxia. Cerebral Cortex. 2005;15(10):1561–1569. doi: 10.1093/cercor/bhi034. [DOI] [PubMed] [Google Scholar]
- Keele SW. Movement control in skilled motor performance. Psychological Bulletin. 1968;70(6, Pt.1):387–403. doi: 10.1037/h0026739. [DOI] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: The importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience. 2003;15(1):30–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, Tranel D. Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex. 2012;48(1):826–848. doi: 10.1016/j.cortex.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kimberg D, Aguirre G, editors. The Human Brain Project/NeuroInformatics Conference Abstract. Bethesda, MD: 2001. VoxBo: A flexible architecture for functional neuroimaging. [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nature Neuroscience. 1999;2(2):176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Bressler SL, Ding M, Coppola R, Nakamura R. Large-scale visuomotor integration in the cerebral cortex. Cerebral Cortex. 2007;17(1):44–62. doi: 10.1093/cercor/bhj123. [DOI] [PubMed] [Google Scholar]
- Lee C, Middleton E, Mirman D, Kalenine S, Buxbaum LJ. Incidental and context-responsive activation of structure- and function-based action features during object identification. Journal of Experimental Psychology: Human Perception and Performance. 2012 doi: 10.1037/a0027533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Mirman D, Buxbaum LJ. Abnormal dynamics of activation of object use information in apraxia: Evidence from eyetracking. Neuropsychologia. 2014;59:13–26. doi: 10.1016/j.neuropsychologia.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Manuel AL, Radman N, Mesot D, Chouiter L, Clarke S, Annoni JM, Spierer L. Inter- and intrahemispheric dissociations in ideomotor apraxia: A large-scale lesion-symptom mapping study in subacute brain-damaged patients. Cerebral Cortex. 2013;23(12):2781–2789. doi: 10.1093/cercor/bhs280. [DOI] [PubMed] [Google Scholar]
- McKinstry C, Dale R, Spivey MJ. Action dynamics reveal parallel competition in decision making. Psychological Science. 2008;19(1):22–24. doi: 10.1111/j.1467-9280.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mirman D, Graziano KM. The neural basis of inhibitory effects of semantic and phonological neighbors in spoken word production. Journal of Cognitive Neuroscience. 2013;25(9):1504–1516. doi: 10.1162/jocn_a_00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A, Simon HA. Human Problem Solving. Englewood Cliffs, NJ: Prentice-Hall; 1972. [Google Scholar]
- Nichols TE, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nickels L, Howard D. Aphasic naming: What matters? Neuropsychologia. 1995;33(10):1281–1303. doi: 10.1016/0028-3932(95)00102-9. [DOI] [PubMed] [Google Scholar]
- Orban GA, Caruana F. The neural basis of human tool use. Frontiers in Psychology. 2014;5:310. doi: 10.3389/fpsyg.2014.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiurak F, Jarry C, Allain P, Aubin G, Etcharry-Bouyx F, Richard I, Le Gall D. Unusual use of objects after unilateral brain damage. The technical reasoning model. Cortex. 2009;45(6):769–783. doi: 10.1016/j.cortex.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Osiurak F, Roche K, Ramone J, Chainay H. Handing a tool to someone can take more time than using it. Cognition. 2013;128(1):76–81. doi: 10.1016/j.cognition.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Oztop E, Arbib MA. Schema design and implementation of the grasp-related mirror neuron system. Biological Cybernetics. 2002;87(2):116–140. doi: 10.1007/s00422-002-0318-1. [DOI] [PubMed] [Google Scholar]
- Pastor-Bernier A, Cisek P. Neural correlates of biased competition in premotor cortex. The Journal of Neuroscience. 2011;31(19):7083–7088. doi: 10.1523/JNEUROSCI.5681-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrims B, Olivier E, Andres M. Dissociation between manipulation and conceptual knowledge of object use in the supramarginalis gyrus. Human Brain Mapping. 2011;32(11):1802–1810. doi: 10.1002/hbm.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: A specific disruption in visuomotor mechanisms: I. Different aspects of the deficit in reaching for objects. Brain. 1988;111(3):643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Poizner H, Mack L, Verfaellie M, Gonzalez Rothi LJ, Heilman KM. Three-dimensional computergraphic analysis of apraxia. Brain. 1990;113(1):85–101. doi: 10.1093/brain/113.1.85. [DOI] [PubMed] [Google Scholar]
- Ramayya AG, Glasser MF, Rilling JK. A DTI investigation of neural substrates supporting tool use. Cerebral Cortex. 2010;20(3):507–516. doi: 10.1093/cercor/bhp141. [DOI] [PubMed] [Google Scholar]
- Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. Different left brain regions are essential for grasping a tool compared with its subsequent use. NeuroImage. 2010;53(1):171–180. doi: 10.1016/j.neuroimage.2010.06.038. [DOI] [PubMed] [Google Scholar]
- Randerath J, Li Y, Goldenberg G, Hermsdörfer J. Grasping tools: Effects of task and apraxia. Neuropsychologia. 2009;47(2):497–505. doi: 10.1016/j.neuropsychologia.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Experimental Brain Research. 2003;153(2):146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Raymer AM, Heilman KM. Limb praxis assessment. In: Rothi LJG, Heilman KM, editors. Apraxia: The Neuropsychology of Action. Hove, UK: Psychology Press; 1997. pp. 61–74. [Google Scholar]
- Rothi LJG, Raymer AM, Ochipa C, Maher LM, Greenwald ML, Heilman KM. Florida apraxia battery: Experimental edition 1991 [Google Scholar]
- Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y, Tsutsui K. Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1998;353(1373):1363–1373. doi: 10.1098/rstb.1998.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54(2):199–227. doi: 10.1016/j.jml.2005.10.002. [DOI] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proceedings of the National Academy of Sciences. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, Wurm MF, Wittmann MK, von Cramon DY. Objects tell us what action we can expect: dissociating brain areas for retrieval and exploitation of action knowledge during action observation in fMRI. Frontiers in Psychology. 2014;5:636. doi: 10.3389/fpsyg.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D'Esposito M. Neural evidence for representation-specific response selection. Journal of Cognitive Neuroscience. 2003;15(8):1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients' privacy rights with researchers' need for access. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain: A Journal of Neurology. 2012;135(Pt 12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey MJ. The continuity of mind. New York: Oxford University Press; 2007. [Google Scholar]
- Spivey MJ, Dale R. Continuous dynamics in real-time cognition. Current Directions in Psychological Science. 2006;15(5):207–211. doi: 10.1111/j.1467-8721.2006.00437.x. [DOI] [Google Scholar]
- Thompson-Schill SL, Botvinick MM. Resolving conflict: A response to Martin and Cheng (2006) Psychonomic Bulletin & Review. 2006;13(3):402–408. doi: 10.3758/BF03193860. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Howard LA, Houghton G. Behavioural consequences of selection from neural population codes. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 223–45. [Google Scholar]
- Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: Representation of action in human anterior intraparietal sulcus. NeuroImage. 2007;36:T77–T86. doi: 10.1016/j.neuroimage.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G. Knowing about tools: Neural correlates of tool familiarity and experience. NeuroImage. 2008;40(3):1380–1391. doi: 10.1016/j.neuroimage.2007.12.058. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Acke F, Vandemaele P, Achten E. Tool responsive regions in the posterior parietal cortex: Effect of differences in motor goal and target object during imagined transitive movements. NeuroImage. 2009;47(4):1832–1843. doi: 10.1016/j.neuroimage.2009.05.100. [DOI] [PubMed] [Google Scholar]
- Watson CE, Buxbaum LJ. Uncovering the architecture of action semantics. Journal of Experimental Psychology: Human Perception and Performance. 2014;40(5):1832–1848. doi: 10.1037/a0037449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Cardillo ER, Ianni GR, Chatterjee A. Action concepts in the brain: An activation likelihood estimation meta-analysis. Journal of Cognitive Neuroscience. 2013;25(8):1191–1205. doi: 10.1162/jocn_a_00401. [DOI] [PubMed] [Google Scholar]
- Watson JDG, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cerebral Cortex. 1993;3(2):79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Conflict and non-conflict tool photographs used as experimental stimuli (photographs from the BOSS database, Brodeur et al., 2010). Tools included in the highly-matched subset are marked with an asterisk.


