Abstract
Pain and alcohol use are both highly prevalent in the general population, and pain-alcohol interrelations are of increasing empirical interest. Previous research has identified associations between pain and alcohol dependence, and the current review provides novel contributions to this emerging domain by incorporating studies that have tested relations between pain and low-to-moderate alcohol consumption, and by identifying potential psychosocial mechanisms of action. Specifically, we sought to integrate evidence of pain-alcohol relations derived from two directions of empirical inquiry (i.e., effects of alcohol on pain and effects of pain on alcohol use) across psychological, social, and biological literatures. We observed converging evidence that associations between alcohol consumption and pain may be curvilinear in nature. Whereas moderate alcohol use was observed to be associated with positive pain-related outcomes (e.g., greater quality of life), excessive drinking and alcohol use disorder appear to be associated with deleterious pain-related outcomes (e.g., greater pain severity). We also observed evidence that alcohol administration confers acute pain-inhibitory effects, and that situational pain may motivate alcohol consumption (e.g., drinking for pain-coping). Future research can inform theoretical and clinical applications through examination of temporal relations between pain and alcohol consumption, tests of hypothesized mechanisms, and the development of novel interventions.
Keywords: pain, alcohol, alcohol use disorder, drinking, chronic pain
Introduction
Interrelations between pain and alcohol are of increasing empirical interest, and research in this area has been identified as a funding priority by the National Institutes of Health. However, progress in this emerging domain has been impeded by mixed findings observed across epidemiological, clinical, and experimental studies, as well as by variability in operational definitions of both pain and alcohol consumption. Studies designed to examine relations between pain and alcohol use can be usefully divided into two directions of empirical inquiry: (1) the effects of alcohol on pain and (2) the effects of pain on alcohol consumption. Although most published studies have focused on either of these directions in isolation, there is increasing interest in research that serves to integrate these findings. In a seminal review on the topic of chronic pain and alcohol use disorder, Egli, Koob, & Edwards (2012) provide evidence that chronic pain and alcohol dependence share overlapping neural substrates and propose a model by which repeated episodes of both pain and alcohol intoxication/withdrawal may result in pathological changes to neural structure and function.
The goal of the current review was to integrate evidence derived from relevant psychological, social, and biological empirical literatures to generate testable hypotheses that may inform future research and the development of novel interventions. The current review extends previous work by examining associations between pain and various levels of alcohol consumption (including low-to-moderate levels of drinking), and by identifying psychosocial mechanisms that may underlie these relations. We begin by providing an overview of the background and terminology relevant to the study of both pain and alcohol, reviewing data regarding the co-occurrence of pain and alcohol use disorder, and discussing potential confounds and relevant third variables. We then review studies examining both the effects of alcohol on pain and the effects of pain on alcohol use, and integrate these findings to conceptualize a series of reciprocal interrelations between pain and alcohol. Finally, we outline directions for future research.
Study Selection and Evaluation
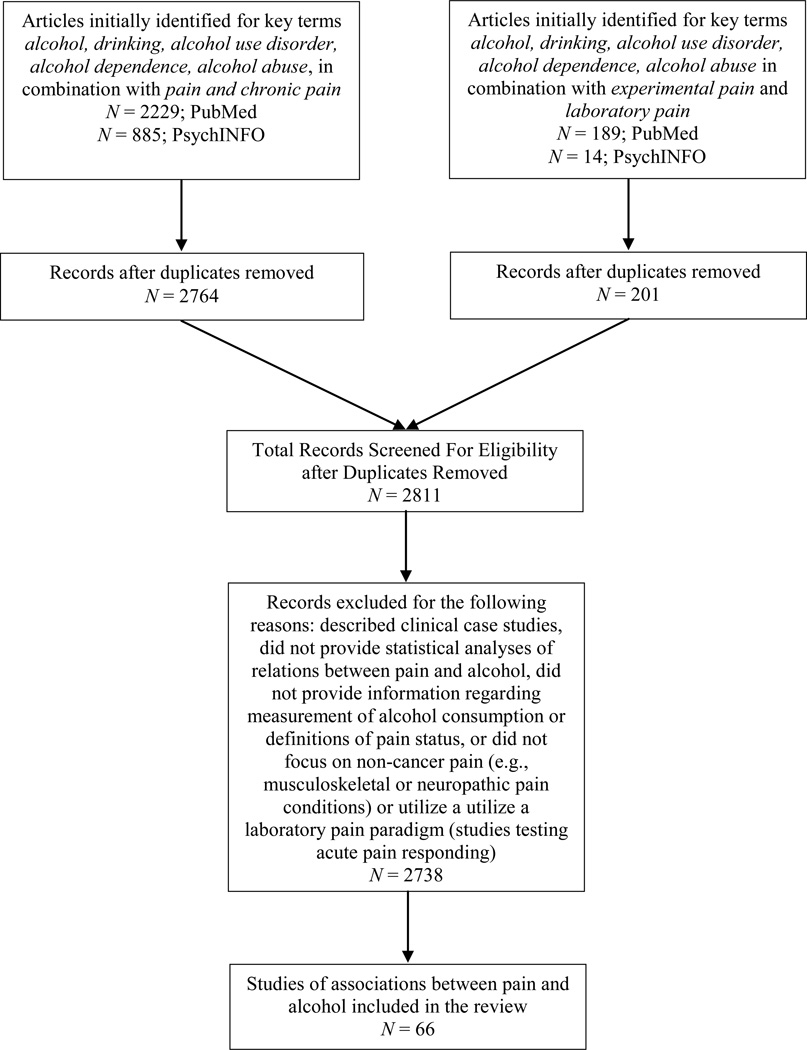
Several converging approaches were employed to identify relevant articles for the current review. First, we conducted computer database searches of PubMed and PsychINFO. Studies examining covariation of pain and alcohol use, associations between alcohol use and pain characteristics, and pain as a motivator of alcohol urge/consumption were identified using the following key search terms in the title or abstract: alcohol, drinking, alcohol use disorder, alcohol dependence, alcohol abuse, in combination with the terms pain and chronic pain. Additional studies examining acute analgesic effects of alcohol were identified using the following key terms: alcohol, drinking, alcohol use disorder, alcohol dependence, alcohol abuse in combination with the terms experimental pain and laboratory pain. Searches were limited to full text papers that were published in English in peer-reviewed journals prior to July 2014. Returned references were then combined and duplicates were removed, resulting in a total of 2811 unique citations, 66 of which were included in the current review (see Figure 1 for a flowchart). Searches were conducted by two independent raters, who also examined reference lists to identify additional relevant citations. Studies were excluded if they described clinical case studies, did not provide statistical analyses of relations between pain and alcohol, did not provide information regarding measurement of alcohol consumption or definitions of pain status, did not focus on non-cancer pain (e.g., musculoskeletal or neuropathic pain conditions), or did not utilize a laboratory pain paradigm (for studies testing acute pain responding). Studies that did not provide direct tests of relations between pain and alcohol were included if relevant to hypothesized mechanisms of action (e.g., social facilitation effects of alcohol; Sayette et al., 2012). Given the broad nature of the research question and the heterogeneity of returned studies, a narrative approach was selected to facilitate the integration of findings derived from studies that utilized experimental (k = 24), prospective (k = 27), cross-sectional (k = 50), or meta-analytic or review (k = 38) methods.
Figure 1.
Flow-chart depicting the study selection process.
Throughout our review of the literature, we noted factors relevant to study quality, including sample size, study design (e.g., longitudinal, cross-sectional, experimental), sample characteristics, variations in measurement of pain and alcohol consumption, and assessment and statistical control for relevant third variables. Accordingly, we include information pertaining to the strengths and limitations of individual studies as they are discussed within the current review. Greater emphasis was given to studies that utilized more rigorous designs (e.g., one study utilized a randomized, double-blind, placebo-controlled experimental design to investigate analgesic effects of alcohol; Perrino et al., 2008), and we note instances in which individual study findings were either interpreted with caution or were less heavily weighted in our conclusions (e.g., seven studies were conducted in older adults and may not generalize to other age groups). Finally, we propose future research directions that were directly informed by our assessment of the strengths and limitations of the extant empirical literature.
Overview of Pain and Alcohol
Acute and Chronic Pain
Pain has been defined as an aversive sensory and emotional experience associated with actual or potential tissue damage (IASP, 1994). Pain has also been described as an inherently subjective experience that encompasses sensory-physiological (e.g., pain intensity), motivational-affective (e.g., pain unpleasantness), and cognitive-evaluative (e.g., pain-related expectancies) dimensions (Turk & Melzack, 2011). Although acute and chronic pain have typically been distinguished by duration (e.g., pain lasting greater than three months considered to be “chronic”), more recent approaches consider a variety of features (e.g., pain intensity, disability) in classifying chronic pain status (Von Korff, 2011). However, there remains little consensus regarding the differentiation of acute vs. chronic pain, with most studies employing cut-offs of pain lasting between 3–12 months (e.g., Turk & Okifuji, 2001).
Despite variability in cut-offs used to define chronic pain, estimates indicate that chronic pain affects approximately 22%-43% of American adults (IOM, 2011; Gureje, Von Korff, Simon, & Gater, 1998) and is responsible for an estimated economic burden of greater than $600 billion in annual healthcare costs and lost productivity (IOM, 2011). In addition to physical impairment and disability, chronic pain has been associated with increased unemployment and absence from work (Braden, Zhang, Zimmerman, & Sullivan, 2008), social isolation and loneliness (Loyland, Miaskowski, Paul, Dahl, & Rustoen, 2010), and increased rates of psychiatric disorders and suicidality (Ilgen, Zivin, McCammon, & Valenstein, 2008; Von Korff et al., 2005).
Alcohol Use
Alcohol use has been defined by a variety of criteria, including dichotomous (e.g., current vs. never/past), continuous (e.g., frequency of use), and categorical (e.g., problem drinking vs. non-problem drinking) classifications (e.g., Bergman, Herrstrom, Jacobsson, & Petersson, 2002; Kim et al., 2013; Kondo et al., 2007). Government agencies have attempted to establish criteria to distinguish levels of moderate drinking (i.e., below a given cut-off) from levels of alcohol consumption that may be considered hazardous, though these criteria tend to vary by country (Dawson, 2000). The United States Department of Health and Human Services (2010) defines excessive alcohol use in terms of either heavy drinking (i.e., >3 drinks on any day or >7 drinks per week for women; and >4 drinks on any day or >14 drinks per week for men) or binge drinking (i.e., >4 drinks in 2 hours for women; and >5 drinks in 2 hours for men). Problem drinking has been defined in terms of negative consequences associated with alcohol consumption, including failure to fulfill obligations at work or school, and interpersonal difficulties (e.g., Finney, Moos, & Brennan, 1991). Diagnostic criteria for Alcohol Use Disorder (AUD) require a maladaptive pattern of alcohol consumption and continued use despite impairment in functioning (see Table A.1 in the appendix for Diagnostic and Statistical Manual criteria).
Nearly half of all US adults report drinking alcohol at least once per month (Schiller, Lucas, & Peregoy, 2012), and up to 30% of adults in the general population have met diagnostic criteria for AUD at some point in their lifetime (Hasin, Stinson, Ogburn, & Grant, 2007). Approximately 100,000 annual US deaths are attributed to alcohol (CDC, 2012a), with an estimated economic impact of greater than $220 billion in healthcare expenditures, lost productivity, and criminal justice costs (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011).
Estimates of Co-Occurring Pain and Alcohol Use
Base rates (i.e., the prevalence of a condition within a given population) may vary as a function of the operational definition of the construct or the population under study (McCaffrey, Duff, & Westervelt, 2000). Perhaps not surprisingly, the base rates of co-occurring pain and alcohol use are difficult to quantify due to: (1) variation in operational definitions of acute/chronic pain and alcohol use, (2) variation in settings from which study samples were derived, and (3) the extent to which confounding factors were measured and/or accounted for. Despite these limitations, researchers have previously utilized data derived from epidemiological, clinical, or setting-specific samples to estimate base rates of co-occurring pain and substance use (e.g., Ditre, Brandon, Zale, & Meagher, 2011).
In a study of persons seeking treatment for substance use disorder, 73% of patients who identified alcohol as their drug of choice also reported moderate-to-severe past-month pain during at least one assessment, with 24% of those reporting past-month pain at all five assessment time-points (i.e., every six months) during the 2-year study period (Larson et al., 2007). Similarly, in a study of community-dwelling older adults, the prevalence of moderate-to-severe past-month pain among problem drinkers (43%) was greater than that observed among non-problem drinkers (30%; Brennan, Schutte, & Moos, 2005). In addition, up to 25% of treatment-seeking pain patients have endorsed heavy drinking (Kim et al., 2013; Lawton & Simpson, 2009), and epidemiological estimates indicate that persons in the general population who endorse back or neck pain (vs. no back or neck pain) may be up to two times more likely to meet diagnostic criteria for AUD (Von Korff et al., 2005). Considering that alcohol use is contraindicated for use of prescription analgesics (FDA, 1998), it is possible that rates of heavy drinking may have been suppressed among some samples, perhaps because patients who use pain medications may be reluctant to report concurrent use of alcohol (e.g., Kim et al., 2013).
Other Factors Associated with Pain and Alcohol Use
Identifying and accounting for potential third variables is essential in the study of co-occurring pain and substance use. Our review of the literature identified a range of biopsychosocial factors and health-related behaviors (e.g., tobacco use, illicit drug use) that may covary with both alcohol use and pain. For example, although we noted that many studies statistically-controlled for some common sociodemographic factors (e.g., age), there was substantial variation in the number of covariates accounted for across studies. In the following section, we briefly examine a selection of biopsychosocial factors that are relevant to both pain and alcohol use.
Both pain and alcohol consumption have been shown to vary across sociodemographic characteristics, including gender, age, race and ethnicity, and socioeconomic status (SES). For example, women appear to be at increased risk for developing a number of painful medical conditions (Fillingim, King, Ribeiro-Dasilva, Rahim-Williams, & Riley, 2009), and even though AUD tends to be more prevalent among men (Hasin et al., 2007), women tend to experience more adverse effects of alcohol consumption (e.g., greater suseptibility to liver disease, greater cognitive and motor impairment due to alcohol consumption; Nolen-Hoeksema, 2004). There is also evidence that men are more likely to drink alcohol to cope with stress (Esper & Furtado, 2013), and men have also been shown to endorse drinking to cope with pain (Brennan et al., 2005; Riley, Gilbert, & Heft, 2002). Although aging has generally been associated with decreased alcohol consumption (e.g., Shaw, Krause, Liang, & McGeever, 2011), there is some evidence to suggest that older adults who experience painful medical conditions may experience drinking problems more often than older adults without painful medical conditions (Brennan, Schutte, SooHoo, & Moos, 2011; Brennan & Soohoo, 2013), and the likelihood of experiencing chronic pain has been shown to increase with age (Tsang et al., 2008). With regard to race and ethnicity, relative to Caucasians, African-Americans who seek treatment for chronic pain tend to endorse more persistent pain and greater unpleasantness (e.g., Edwards, Fillingim, & Keefe, 2001). There is also evidence that African-Americans may be at greater risk for recurring AUD, relative to Caucasians (Chartier & Caetano, 2010). Finally, among persons with chronic pain, lower SES has been associated with greater disability and more severe pain (e.g., Green & Hart-Johnson, 2012). AUD is also more common among persons with lower incomes (Hasin et al., 2007), and low SES has been associated with greater quantities of alcohol consumption (Huckle, You, & Casswell, 2010).
In addition to sociodemographic characteristics, biopsychosocial factors including body mass index (BMI), co-occurring anxiety and depressive disorders, and familial/genetic contributions have been individually associated with both pain and alcohol consumption. First, greater BMI, which may serve as a marker for obesity (CDC, 2011), has been identified as a risk factor for chronic low-back pain, pain-related disability, and reduced quality of life (e.g., Heuch, Hagen, & Zwart, 2013; Marcus, 2004). There is also evidence that persons who are classified as “obese” are more likely to meet diagnostic criteria for AUD (Petry, Barry, Pietrzak, & Wagner, 2008). Second, symptoms of both anxiety and depression are highly prevalent among persons with chronic pain (e.g., Dersh, Polatin, & Gatchel, 2002), and AUD (e.g., Hasin et al., 2007), relative to the general population. Furthermore, comorbid psychiatric disorders have been associated with greater pain-related disability among persons with chronic pain (Dersh, Polatin, & Gatchel, 2002), and there is some evidence that AUD may serve to maintain symptoms of depression and anxiety (Boschloo et al., 2012). Finally, family history of chronic pain has been associated with a greater likelihood of developing chronic pain (Hoftun, Romundstad, & Rygg, 2013), and both shared genetic and environmental factors have been implicated in this progression (Kato, Sullivan, Evengard, & Pedersen, 2006). The prevalence of AUD is greater among persons with a family history of AUD (e.g, Grant, 1998), and genetic contributions to AUD have consistently been observed (e.g., Ehlers, Walter, Dick, Buck, & Crabbe, 2010). Notably, a family history of AUD has also been observed among chronic pain patients (Pecukonis, 2004).
Both alcohol consumption and pain have been associated with health-related behaviors, including use of illicit substances and tobacco smoking. For example, substance use disorders appear to be highly prevalent among both persons seeking pain treatment (Manchikanti et al., 2006) and persons with AUD (Stinson et al., 2005). In addition, the prevalence of tobacco smoking among persons with chronic pain (49%–68%; Hooten, Shi, Gazelka, & Warner, 2011; Michna et al., 2004) and AUD (90%; Bien & Burge, 1990) appears to be substantially greater than that observed in the general population (19%; CDC, 2012b). There is also evidence to suggest that alcohol use may be more common among smokers (relative to nonsmokers) receiving multidisciplinary pain treatment (Fishbain et al., 2007).
Effects of Alcohol on Pain
In this section, we review evidence that (1) associations between alcohol consumption and the development and characteristics of chronic pain may be curvilinear in nature, (2) alcohol consumption may be associated with pain-treatment complications, (3) acute alcohol administration may have short-term pain-inhibitory effects, and (4) both humans and animals may demonstrate increased pain responding (i.e., hyperalgesia) in the context of alcohol abstinence and withdrawal.
Evidence of Curvilinear Associations between Alcohol Consumption and Pain Conditions
A growing body of evidence suggests that excessive alcohol consumption may be associated with the onset and severity of numerous painful conditions. First, heavy alcohol consumption is an established causal agent in the development of alcohol-induced pancreatitis, possibly via physiological pathways that cause tissue damage and changes to digestive processes (Lerch et al., 2003). Pancreatitis is an inflammatory disease of the pancreas, which can be acute (i.e., sudden onset, short duration) or chronic (i.e., gradual onset, increased severity over time) in nature, and can cause severe abdominal pain (Banks, Conwell, & Toskes, 2010). In the United States, alcohol accounts for approximately 33% of acute, and 60%–90% of chronic pancreatitis cases (Dufour & Adamson, 2003). Alcohol-induced pancreatitis (vs. other etiologies) is a risk factor for more severe pain intensity, decreased quality of life, and greater disability (Mullady et al., 2011), and the majority of persons with alcohol-induced pancreatitis experience severe pain that results in hospitalization (Ammann & Muellhaupt, 1999).
Excessive alcohol consumption is also a known causal agent in the development of alcohol-related neuropathy, which can be characterized by damage to sensory, motor, and autonomic nerves, potentially due to direct neurotoxic effects of alcohol on the central and peripheral nervous systems (e.g., Chopra & Tiwari, 2012). Pain is a predominant and early feature of alcohol-related neuropathy, and treatment typically requires both acute and long-term pain management (Chopra & Tiwari, 2012; Njamnshi & Wisysonge, 2010). The estimated prevalence of alcohol-related neuropathy is 25%–66% among persons who meet criteria for AUD (Chopra & Tiwari, 2012).
Additionally, there is reason to believe that excessive alcohol consumption may be associated with the onset and severity of other chronic pain conditions (see Table 1). For example, the results of a large prospective study (N = 16,961, M follow-up time = 10.9 years) revealed that men who endorsed consuming greater than 14 drinks per week (vs. no alcohol use) at baseline were more likely to develop osteoarthritis, even after controlling for BMI, tobacco smoking, age, physical activity, caffeine intake, and length of follow-up (hazard ratio [HR] = 1.4; 95% CI [1.1, 1.8]; Cheng et al., 2000). Similar results were observed in two population-based cross-sectional studies, which demonstrated that daily alcohol consumption and frequent intoxication were associated with increased likelihood of reporting recurrent knee pain (Odds Ratio [OR] = 6.85; 95% CI [1.55, 30.31]; Sá, Baptista, Matos, & Lessa, 2008), and chronic pain lasting greater than 6 months among women (OR = 1.83; 95% CI [1.00, 3.33]; Sá, de Mesquita Pereira, Souza, Baptista, & Lessa, 2011).
Table 1.
Summary of Studies that Tested the Relation between Alcohol and Chronic Pain
| Study | Design | N | Population | Alcohol Use | Pain Outcome | Covariates | Main Findings |
|---|---|---|---|---|---|---|---|
| Positive effects of alcohol | |||||||
| Ang et al. (2006) | P (5-year) | 370 | Gulf War Veterans | Yes/No | CWP (Yes/No) | Age, gender, service branch | Alcohol use predicted decreased likelihood of developing CWP |
| Bergman et al. (2002) | P (3-year) | 2,425 | Community-based survey | Weekly Alcohol Use (vs. no use) | CWP (Yes/No) | Age, sex, SES, immigrant status, housing area, education, smoking, personal support, family history of chronic pain, pain at baseline | Weekly alcohol use predicted decreased likelihood of developing CWP |
| Booker et al. (2003) | CS | 283 | Back pain patients | Drinks/Week | Laboratory physical functioning | None | Men: >12 drinks/week was associated with improved physical performance |
| Gorman et al. (1987) | CS | 142 | LBP clinic patients | Alcohol use at least monthly (vs. no use) | Pain-related disability | None | Alcohol use at least monthly was associated with lower disability |
| Hestbaek et al. (2006) | P (8-year) | 6,554 | Danish Twin Registry | Yes/No | Persistent LBP (Yes/No) | Age, sex | Alcohol use predicted decreased likelihood of developing persistent LBP |
| Kim et al. (2013) | CS | 946 | Fibromyalgia patients | Drinks/week | Fibromyalgia symptoms, quality of life, missed work | Age, employment, education, BMI, opioid use | < 7 drinks/week was associated with improved quality of life, reduced symptoms, less missed work |
| Kondo et al. (2007) | CS | 109 | Male patients diagnosed with RA | Yes/No | Functional limitations | Age, height, weight, smoking, sitting posture, occupation, previous knee pain and/or swelling | Alcohol use was associated with fewer functional limitations |
| Rivera et al. (2008) | P (1-year) | 3,047 | Patients with traumatic injuries | Non-drinker (vs. non-hazardous or hazardous drinker) | Pain severity related to injury | None | Non-drinkers reported slightly greater pain severity |
| Sa et al. (2008) | CS | 2,297 | Population-based survey | Non-daily alcohol use (vs. no use) | CP > 6 months (Yes/No) | Age, marital status, smoking, obesity | Women: Non-daily alcohol use was associated with decreased likelihood of CP Men: NS |
| Sa et al. (2011) | CS | 2,297 | Population-based survey | Non-daily alcohol use (vs. no use) | Recurrent knee pain (Yes/No) | None | Non-daily alcohol use was associated with decreased likelihood of knee pain |
| Skillgate et al. (2009) | P (3-year) | 6,532 | Swedish Employment Registry | Alcohol use > once/month (vs. no use) | Long-term sick-leave for back or neck pain | None | Alcohol use > than once/month predicted decreased likelihood of registering for sick leave |
| Negative Effects of Alcohol | |||||||
| Castillo et al. (2006) | P (7-year) | 397 | Lower limb trauma patients | Drinks/day | Chronic pain grade | Education, injury type, treatment type, anxiety & depression symptoms, pain intensity, self-efficacy, narcotic mediation use | >2 drinks/day predicted greater chronic pain grade |
| Cheng et al. (2000) | P (varied) | 16,961 | Patients at an outpatient clinic | Drinks/week | Physician-diagnosed osteoarthritis at follow up | BMI, smoking, age, physical activity, caffeine intake, time of follow-up | Men: >14 drinks/week increased likelihood of developing osteoarthritis |
| Holmes et al. (2010) | P (3-month) | 242 | Trauma center patients | AUD (vs. no AUD) | Pain severity related to injury | Anxiety and depression | History of AUD was associated with greater pain at follow up |
| Sa et al. (2008) | CS | 2,297 | Population-based survey | Frequent intoxication/daily consumption (vs. no use) | Age, sex, obesity, marital status, physical activity | Frequent intoxication/daily consumption more likely to endorse knee pain | |
| Sa et al. (2011) | CS | 2,297 | Population-based survey | Frequent intoxication/daily consumption (vs. no use) | age, marital status, smoking, obesity | Women: Frequent intoxication/daily consumption more likely to endorse CP Men: NS |
|
| Null Findings | |||||||
| Helmer et al. (2009) | CS | 429 | OEF/OIF Veterans | Problem drinking (vs. non-problem drinking) | CWP (Yes/No) | None | NS |
Note. P = prospective; CS = cross-sectional; CWP = chronic widespread pain; LBP = low-back pain; CP = chronic pain; RA = Rheumatoid Arthritis; Chronic pain grade = severity and pain-related interference in functioning; NS = not statistically significant at p < .05; OEF/OIF = Operation Enduring Freedom and Operation Iraqi Freedom.
There is also evidence that excessive alcohol consumption may predict poorer pain-related outcomes following traumatic injury. For example, the results of a multicenter study indicated that persons with lower limb trauma who endorsed consuming greater than two drinks per day (vs. less than two drinks per day) were more likely (56% vs. 36%; χ2 = 7.8, p = .006) to develop severe chronic pain, and that excessive alcohol consumption remained a significant predictor of chronic pain severity even after controlling for sociodemographic, injury-related, and mental-health characteristics (Castillo, MacKenzie, Wegener, Bosse, & Group, 2006). Similar results were observed among patients admitted to a trauma center, such that those with a history of AUD at baseline went on to report greater pain severity at 3-month follow-up, independent of comorbid anxiety and depressive symptoms (Holmes et al., 2010). Although one cross-sectional study showed no association between problem drinking and chronic widespread pain (Helmer et al., 2009), this finding should be interpreted with caution, as the sample was limited to veterans referred for evaluation related to post-deployment concerns (e.g., mental health symptoms), and because these analyses did not account for relevant third variables.
Excessive alcohol consumption may be associated with poorer pain outcomes through a variety of mechanistic pathways. First, according to the allostatic load model of pain and AUD (Egli et al., 2012), pathological changes to neural structures (e.g., central amygdala, prefrontal cortex, insula, nucleus accumbens) and function may contribute to the development of chronic pain among persons with AUD. For example, this model posits that excessive alcohol consumption may contribute to the development and exacerbation of chronic pain via dysregulation of the endogenous opioid system, which plays a role in both pain modulation and the reinforcing effects of alcohol. Whereas acute administration of alcohol has been shown to stimulate the release of opioid peptides, long-term, higher-level alcohol exposure may result in central opioid deficiency (Gianoulakis, 2001). Indeed, deficiencies in endogenous pain modulation appear to be common across pain conditions and have been implicated in both the onset and progression of chronic pain (e.g., Edwards, 2005). Researchers have also proposed that excessive drinking may contribute to the development of chronic pain via increased risk of traumatic injury and deleterious effects on the musculoskeletal system (Brennan et al., 2011; Brennan & Soohoo, 2013; Govindu & Babski-Reeves, 2013; Sá et al., 2011).
In contrast to evidence that excessive alcohol consumption may be associated with the onset and exacerbation of chronic pain, there is initial evidence from well-designed population studies, which controlled for a host of relevant third variables (e.g., sociodemographic characteristics, family history of chronic pain), that low-to-moderate alcohol consumption may be associated with a decreased likelihood of developing chronic pain (see Table 1). For example, two separate longitudinal studies demonstrated that persons who endorsed any alcohol use (vs. no alcohol use) were 80% less likely to develop chronic widespread pain (Ang, Peloso, Woolson, Kroenke, & Doebbeling, 2006) and 25% less likely to develop persistent low-back pain (Hestbaek, Leboeuf-Yde, & Kyvik, 2006). Additionally, two prospective studies demonstrated that moderate alcohol consumption (vs. no consumption) was associated with a 40%–70% reduced likelihood of developing disabling chronic back or neck pain (Skillgate, Vingard, Josephson, Holm, & Alfredsson, 2009) and chronic widespread pain (Bergman et al., 2002). Finally, several cross-sectional findings have demonstrated associations between moderate alcohol consumption (vs. no consumption) and decreased likelihood of endorsing recurrent pain (OR = 0.68; 95% CI [0.25, 0.87]; Sá et al., 2008), and chronic pain lasting greater than 6 months among women (OR = 0.63; 95% CI [0.47, 0.85]; Sá et al., 2011).
There is also some evidence that moderate alcohol users (relative to non-drinkers) may experience better pain outcomes. One large-scale study conducted among treatment-seeking pain patients with fibromyalgia demonstrated that low-to-moderate alcohol consumption (vs. no consumption) was associated with greater quality of life and physical functioning, fewer fibromyalgia symptoms, and less missed work, even after controlling for sociodemographic characteristics, BMI, and medication use (Kim et al., 2013). Similar results have been observed among patients with rheumatoid arthritis (Kondo et al., 2007) and chronic back pain (Gorman et al., 1987), and one study observed slightly lower pain intensity ratings among alcohol users (vs. nonusers) following traumatic injury (Rivara et al., 2008). Finally, in one study of patients referred for chronic back pain assessment, males who reported drinking 12 or more drinks per week performed better on tests of physical functioning, relative to lighter drinking and abstinent patients (Booker, Haig, Geisser, & Yamakawa, 2003). However, given that alcohol consumption was reported as a categorical variable, it is not possible to know whether some of the participants in that study drank at levels above the cut point for moderate drinking (i.e., > 14 drinks/week for adult males; USDHHS, 2010).
Several mechanisms have been proposed to explain associations between moderate alcohol use and improved pain outcomes. First, fear-avoidance models of chronic pain posit that pain-related fear (e.g., fear of movement, re-injury, or pain) is an important psychological factor associated with the development and course of chronic pain conditions (Leeuw et al., 2007), and meta-analytic estimates indicate a robust association between pain-related fear and disability outcomes (Zale, Lange, Fields, & Ditre, 2013). One possibility is that as alcohol consumption facilitates disinhibition, the salience of fear-avoidance mechanisms may be reduced, which could, in turn, enhance pain-related functioning (Booker et al., 2003). There is also reason to believe that observations of improved quality of life and reduced disability may be related to social integration and stress-relieving effects of alcohol. Alcohol users report social motives for drinking (Kuntsche, Knibbe, Gmel, & Engels, 2005), moderate alcohol consumption has been associated with social integration and sociability (Peele & Brodsky, 2000), and alcohol use appears to facilitate social bonding (Sayette et al., 2012). Given that chronic pain has been associated with social isolation (Loyland et al., 2010), researchers have hypothesized that persons with chronic pain may drink alcohol to facilitate social interactions (Kim et al., 2013). Additionally, chronic pain can be a salient stressor (Blackburn-Munro & Blackburn-Munro, 2001). Given that alcohol users tend to hold expectancies for stress-reduction via drinking (e.g., Ayer, Harder, Rose, & Helzer, 2011), persons with chronic pain who consume alcohol in moderate amounts may do so, in part, to alleviate stress, which could result in greater perceived functioning (Kim et al., 2013).
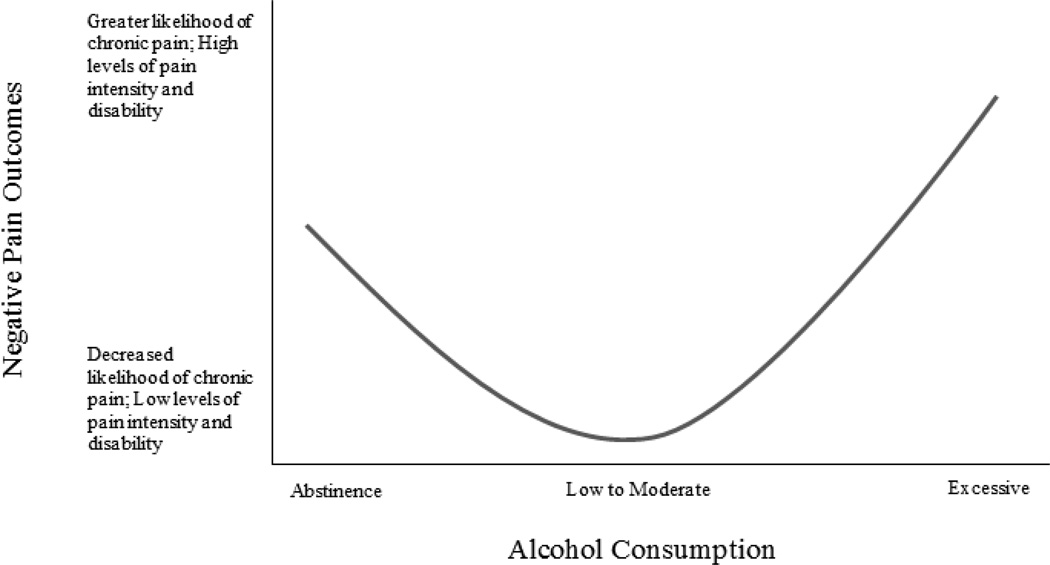
Based on evidence that excessive alcohol consumption may be associated with poorer pain-related functioning and an increased likelihood of developing chronic pain, and that moderate alcohol use (vs. no use) may be associated with better pain-related functioning and a decreased likelihood of chronic pain onset and progression, we conceptualize associations between alcohol consumption and pain as potentially curvilinear in nature (see Figure 2). This conceptualization is consistent with previously observed curvilinear associations between alcohol use and other health outcomes (e.g., Plunk, Syed-Mohammed, Cavazos-Rehg, Bierut, & Grucza, 2013), including previously reviewed associations between alcohol and migraine headache (Panconesi, 2008). Taken together, these findings underscore the importance of obtaining a precise measurement of alcohol consumption (e.g., amount, frequency) in relation to chronic pain onset and severity. Most of the studies that observed associations between alcohol use and reduced pain utilized dichotomous measures (e.g., any alcohol use vs. no use; weekly alcohol use vs. no use), thus precluding a graded specification of the alcohol-pain relation. Similarly, we observed inconsistencies across studies with regard to how excessive alcohol consumption was operationally defined (e.g., more than 2 drinks/day, frequent intoxication). It is, however, encouraging that the majority of studies (k = 9) reviewed herein utilized large sample sizes (Ns ranged from 2,297 – 16,961), and included statistical-controls for a variety of relevant third variables. For example, 11 of the studies reviewed in this section included at least two covariates, and eight studies included both sociodemographic (e.g., age, sex, gender) and health-behavior (e.g., substance use) variables as covariates. Although eight studies tested prospective associations between alcohol use and chronic pain, the follow-up period varied widely (i.e., 3 months – 11 years). Thus, the temporal nature of these relations should be interpreted with caution as variations in follow-up duration may have precluded observation of some pain outcomes (e.g., given variations in the cut-offs used to distinguish acute from chronic pain, 3-month follow-up may be insufficient to detect new onset of chronic pain after injury).
Figure 2.
Hypothesized curvilinear relation between alcohol consumption and chronic pain. Future research is needed to identify the levels of alcohol consumption at which the direction of effects changes (e.g., positive to negative).
Alcohol Use and Pharmacologic Pain Treatments
Alcohol consumption is contraindicated for use of prescription and over-the-counter pain medications, and interactions between alcohol and pain medications may have dangerous health effects (FDA, 1998). Alcohol may (1) increase the risk of gastrointestinal bleeding associated with non-steroidal anti-inflammatory medications, (2) interact with acetaminophen metabolism in a manner that promotes liver damage, and (3) enhance the depressive effects of opioids and benzodiazepines on the central nervous system (Fraser, 1997; Hersh, Pinto, & Moore, 2007). Interactions between alcohol and pain relievers (e.g., narcotics) account for nearly 115,000 emergency room visits annually (SAMHSA, 2012).
Despite these health risks, approximately one third of persons who receive prescription opioids endorse alcohol consumption (36%; Saffier, Colombo, Brown, Mundt, & Fleming, 2007), and AUD may be more prevalent among persons who use prescription opioid medications, relative to persons in the general population (Jobski, Schmid, Behr, Andersohn, & Garbe, 2012). Prescription opioid misuse has been defined as inappropriate use of opioid medication, including aberrant drug behaviors (e.g., dose escalation, use other than as prescribed; Pergolizzi et al., 2012). Multiple reviews have concluded that a history of substance use disorder, including alcohol, is the strongest predictor of opioid misuse (Turk, Swanson, & Gatchel, 2008), and that excessive alcohol consumption appears to precede the onset of opioid misuse (Pergolizzi et al., 2012). Results from animal models also indicate that chronic, alcohol consumption may result in cross-tolerance to the pain-inhibitory effects of opioids (He & Whistler, 2011), and researchers have proposed that hypersensitivity to emotional distress in the context of opioid misuse may contribute to the development and maintenance of chronic pain (Egli et al., 2014).
Short-Term Pain-Inhibitory Effects of Acute Alcohol Administration
Evidence derived from both animal and human studies indicates that acute alcohol administration may confer short-term pain-inhibitory effects. For example, animal models have consistently demonstrated increased pain threshold following acute ethanol administration, with some data suggesting a dose-response effect (e.g., Ibironke & Oyekunle, 2012). These animal models further demonstrated that acute pain-inhibitory effects of alcohol tended to diminish following 10–12 days of ethanol administration, which suggests that short-term analgesic effects may be reduced in the context of chronic alcohol exposure (Gatch & Lal, 1999).
Despite consistent evidence from the animal literature, and well-documented historical use of alcohol as an anesthetic (e.g., Shealy & Cady, 2002), only a few experimental studies have been conducted among humans to test the causal effects of acute alcohol administration on laboratory pain reactivity. Human laboratory pain models allow researchers to mimic signs and symptoms of painful medical conditions without causing lasting damage. Common paradigms include mechanical pressure, electrical stimulation, and exposure to thermal stimuli. Primary outcomes tend to include pain threshold, which is typically defined by the time (e.g., seconds) or stimulus intensity (e.g., volts) at which participants first report pain, and pain tolerance, which represents the duration of exposure or maximum stimulus intensity that a participant is willing to endure (IASP, 1994).
Initial results derived from human laboratory studies suggest that alcohol may confer acute analgesic effects. For example, a randomized, double-blind, placebo controlled cross-over study demonstrated that intravenous alcohol administered at high concentrations (1g/dl) increased pain tolerance (but not threshold) in response to electrical stimulation (Perrino et al., 2008), and that analgesic responding was greater among participants with a family history of AUD who were also high in the personality trait neuroticism (Ralevski, Perrino, Acampora, Koretski, Limoncelli, & Petrakis, 2010). Analgesic effects have also been observed for electric shock pain (Stewart, Finn, & Pihl, 1995) and mechanical pressure pain (Woodrow & Eltherington, 1988) in the context of orally-administered alcohol. Although we identified two additional studies that demonstrated acute analgesic effects of alcohol (James, Duthie, Duffy, McKeag, & Rice, 1978; Wolff, Hardy, & Goodell, 1941), neither utilized an experimentally-rigorous design, and one study (Wolff et al., 1941) was conducted using only the three authors as subjects.
There is also evidence that past experiences with alcohol may moderate acute analgesic effects of alcohol consumption (Brown & Cutter, 1977; Cutter, Maloof, Kurtz, & Jones, 1976). For example, one study demonstrated that consuming alcohol decreased pain ratings only among participants who met diagnostic criteria for AUD or endorsed problem drinking (Cutter, Jones, Maloof, & Kurtz, 1979). Similarly, Brown & Cutter (1977) observed that whereas higher doses of orally-administered alcohol decreased pain intensity ratings among participants who endorsed drinking alone in bars, which was used as a measure of problematic alcohol use, participants who reported only consuming alcohol during social occasions with their families (a proxy for moderate drinking) demonstrated analgesia to lower, but not higher, doses of alcohol. The authors interpreted these results as indicating that customary levels of drinking may provide optimal pain reduction. It is also possible that participants who were considered problem drinkers may have developed a tolerance to alcohol (e.g., Schuckit et al., 2008), which could explain why a higher dose of alcohol was necessary to achieve analgesic effects in that group.
Potential mechanisms of the acute pain inhibitory effects of alcohol include activation of the endogenous opioid system and response expectancies. Acute alcohol administration has been shown to stimulate the release of endogenous opioids (Mitchell et al., 2012), which may contribute to reduced pain perception. Support for the role of endogenous opioids in alcohol-induced analgesia is further supported by animal studies, which consistently demonstrate that acute pain-inhibitory effects of alcohol can be attenuated via administration of opioid antagonists (e.g., Campbell, Taylor, & Tizabi, 2007). There is also evidence that pain-inhibitory effects of alcohol tend to be reduced among mice with lower concentrations of opioid receptors, and augmented among mice with greater concentrations of opioid receptors (Yirmiya & Taylor, 1989). Finally, there is some evidence that analgesic properties of alcohol may be partially mediated by availability of benzodiazepine receptors (Gatch, 1999).
There is also reason to believe that expectancies for pain relief via drinking may have a potent influence on pain reporting (Pollo et al., 2001). Individuals may come to hold beliefs that alcohol will help them manage pain if they have previously perceived a reduction in their pain (or pain-related distress) when drinking. Given evidence that alcohol expectancies may be influenced by socially shared and transmitted beliefs (Donovan, Molina, & Kelly, 2009), it is possible that expectancies for alcohol-induced analgesia may be shaped by social depictions of alcohol as a stress-coping agent. However, we are not aware of any studies that have attempted to assess whether participants held expectancies that drinking may mitigate pain in the context of laboratory pain induction.
Hyperalgesia during Acute Abstinence from Chronic Alcohol Use
In contrast to evidence that acute alcohol administration may confer analgesic effects, converging evidence derived from both animal and human studies suggests that abstinence from chronic alcohol consumption may produce hyperalgesia, which is characterized by increased pain in response to noxious stimuli (IASP, 1994). For example, rodent models of alcohol withdrawal have reliably demonstrated hyperalgesia to multiple pain assays following discontinuation of chronic ethanol administration (for review, see Gatch, 2009). Given these consistent findings, hyperalgesia has been suggested to be a characteristic symptom of alcohol withdrawal in animals (Becker, 2000). Among humans, there is initial evidence that patients receiving treatment for AUD may exhibit greater pain responding during the early stages of alcohol abstinence (Jochum, Boettger, Burkhardt, Juckel, & Bar, 2010). Specifically, following admission for detoxification, patients experiencing acute alcohol withdrawal demonstrated decreased pain tolerance and threshold during laboratory pain induction, relative to both abstinent (2–3 months) patients and healthy controls. Furthermore, among patients undergoing acute detoxification, withdrawal severity was positively associated with greater pain responding, and pain reactivity was significantly decreased at discharge (14 days after admission). These results indicate that hyperalgesia may occur during periods of acute abstinence and withdrawal, and that these effects may subside following approximately two weeks of alcohol abstinence.
A recent review on the topic of alcohol withdrawal and hyperalgesia in animal models identified down-regulation of adenosine receptors, and up-regulation of L-type calcium channels, as likely mediators of alcohol withdrawal-induced hyperalgesia (Gatch, 2009). For example, co-administration of alcohol and theophylline (i.e., an adenosine receptor antagonist) has been shown to attenuate development of hyperalgesia during withdrawal, presumably because theophylline promotes up-regulation of adenosine A1 receptors (Gatch & Selvig, 2002). Co-administration of L-type calcium channel blockers and alcohol has also been shown to reduce hyperalgesia during alcohol abstinence, possibly because L-type calcium channel blockers prevent up-regulation of L-type calcium channels that would otherwise occur in the context of chronic alcohol administration (Gatch, 2009).
Increased pain in the context of alcohol abstinence may be of particular relevance for persons with co-occurring chronic pain and AUD. The fear-avoidance model of chronic pain posits that persons who experience chronic or recurrent pain may be hypervigilant to perceived increases in pain (Leeuw et al., 2007), which suggests that persons with chronic pain may be especially sensitive to hyperalgesia during the early stages of alcohol abstinence. Hyperalgesic responses have been observed during withdrawal from other substances (e.g., nicotine), and researchers have proposed that increased pain may precede relapse (e.g., Ditre et al., 2011). Thus, increased pain in the context of alcohol abstinence and withdrawal may have important clinical implications for the treatment of AUD among persons who experience chronic pain.
Effects of Pain on Alcohol Use
In this section, we review evidence that (1) greater pain severity may be associated with increased drinking problems, (2) co-occurring pain may be associated with poorer alcohol-treatment outcomes, and (3) pain may serve as a situational motivator of alcohol consumption. We also discuss potential mechanisms by which pain may motive alcohol use.
Covariation of Pain Severity, Alcohol Consumption, and Problematic Drinking
There is early, albeit mixed, cross-sectional evidence that specific pain characteristics (e.g., severity, unpleasantness, interference) may be associated with greater problem drinking. For example, in a sample of adults with chronic pain, greater levels of pain intensity and unpleasantness were associated with increased alcohol consumption and rates of hazardous drinking (Lawton & Simpson, 2009). In the same sample, results further indicated that greater pain unpleasantness was associated with more severe alcohol dependence, independent of relevant sociodemographic and mental health factors (Lawton & Simpson, 2009). Similar findings revealed that older adults who endorsed more severe pain and greater pain-related interference with daily activities (compared to those with no pain) were 20% and 50% more likely to also have endorsed current drinking problems, respectively (Brennan & Soohoo, 2013). Among older adults, a greater number of painful medical conditions has been associated with increased frequency of experiencing drinking problems (Brennan et al., 2011). Persons with chronic pain may report greater alcohol consumption and drinking problems if alcohol is used as a means of pain-coping. The self-medication hypothesis (e.g., Khantzian, 1985) posits that substances may be used to reduce negative affect, and a growing body of evidence suggests that pain may serve as a motivator of substance use (e.g., Ditre et al., 2011). Indeed, the use of alcohol to cope with stress is a well-established phenomenon, and drinking for stress-coping has been associated with greater levels of alcohol consumption and frequency of drinking problems (e.g., Holahan, Moos, Holahan, Cronkite, & Randall, 2001).
Conversely, there is some prospective evidence that older adults who endorse more severe pain or a greater number of painful conditions may ultimately go on to reduce their alcohol consumption (Bobo, Greek, Klepinger, & Herting, 2012; Brennan et al., 2011; Brennan & Soohoo, 2013). However, each of these studies sampled older adults who did not necessarily have chronic pain, reported low levels of baseline drinking, and whose patterns of alcohol use may not generalize to other age groups. For example, an age-related decline in alcohol use tends to begin following young adulthood (Shaw et al., 2011), and older adults have evinced a general motivation to reduce alcohol use in response to health concerns (Dawson, Goldstein, & Grant, 2013).
Associations between Pain and Alcohol-Treatment Outcomes
The majority of studies that tested the associations between pain and treatment for substance use disorders have either focused on opioid misuse (e.g., Trafton, Oliva, Horst, Minkel, & Humphreys, 2004), or did not distinguish alcohol from other substances (e.g., Ilgen et al., 2010). However, there is some converging evidence that people with co-occurring pain disorders experience greater difficulty when attempting to abstain from drugs and alcohol (Caldeiro et al., 2008). In addition, among a sample of inpatients seeking treatment for substance use disorders (40% of whom endorsed alcohol as their substance of choice), persistent pain was associated with an increased likelihood of post-treatment alcohol consumption (Larson et al., 2007). There are also pilot data supporting the notion that chronic pain patients may benefit from integrated treatments that concurrently address pain and substance use (Ilgen et al., 2011).
Pain as a Situational Motivator of Alcohol Use
There is cross-sectional evidence that some individuals may be motivated to use alcohol to cope with pain. For example, individuals with co-occurring substance use disorders and chronic pain have cited pain as the primary reason they began misusing alcohol or illicit substances (Sheu et al., 2008). Among chronic pain patients, greater levels of pain unpleasantness have been associated with increased motivation to use alcohol (Lawton & Simpson, 2009). Indeed, the use of alcohol to cope with pain has been endorsed by almost one quarter of all patients enrolled in both pain treatment (24%; Goebel et al., 2011) and inpatient substance abuse programs (22%; Sheu et al., 2008). Similar findings have been observed among community-based samples, with greater severity and frequency of pain shown to be associated with an increased likelihood of using alcohol to manage pain (Arcury, Bernard, Jordan, & Cook, 1996; Riley & King, 2009), possibly more so among men (Riley et al., 2002).
Consistent with cross-sectional evidence of pain as a motivator of alcohol use, one prospective study revealed that persons who endorsed problem drinking at baseline were more likely to have also endorsed using alcohol to cope with pain, and that participants who endorsed use of alcohol for pain-coping at baseline were more likely to increase alcohol consumption over the three-year study period (Brennan et al., 2005). This observation is consistent with evidence that drinking for stress-coping tends to be associated with increased alcohol consumption and a greater number of alcohol-related problems (Holahan et al., 2001). Finally, the use of alcohol to manage pain has been linked with poorer health outcomes, including health quality, medication adherence, and number of chronic health problems (Brennan et al., 2005; Immonen, Valvanne, & Pitkala, 2011).
Potential mechanisms by which pain may serve as a motivator of alcohol use include negative and positive reinforcement, lack of alternative strategies for pain-coping, and overlapping neural systems that process stress and reward. Negative reinforcement models of addiction posit that substance use is motivated, in part, by a desire to alleviate aversive psychological and physical states (e.g., McCarthy, Curtin, Piper, & Baker, 2010). One possibility is that pain may motivate alcohol consumption via a desire to alleviate pain-related negative affect. Negative affect is a central component of pain-processing (e.g., Wade, Dougherty, Archer, & Price, 1996), and it has been suggested that coping with negative affect may be a primary drinking motive among persons with AUD (e.g., Kuntsche et al., 2005). As noted earlier, pain has also been conceptualized as a stressor (Blackburn-Munro & Blackburn-Munro, 2001), and alcohol users may be motivated to drink in response to stress, particularly if they hold expectancies for alcohol-induced tension reduction (Armeli, Carney, Tennen, Affleck, & O'Neil, 2000).
As noted in previous sections, alcohol has been shown to have acute analgesic effects (e.g., Perrino et al., 2008). Therefore, it is possible that some individuals may hold expectancies for pain relief via alcohol consumption. Also, given initial evidence that persons who are alcohol dependent experience increased pain during periods of abstinence (Jochum et al., 2010), it seems reasonable to hypothesize that increased pain may precipitate relapse to drinking. Finally, given that stress-related cues have been shown to increase craving in abstinent alcoholic patients (Sinha et al., 2009), there is reason to hypothesize that pain may increase cravings for alcohol, and that persons in pain may be motivated to drink in order to alleviate those cravings (e.g., Bottlender & Soyka, 2004).
In addition to negative reinforcement pathways, pain may also motivate alcohol consumption via positive reinforcement. According to behavioral activation theory, humans are sensitive to the availability of positive reinforcers (Dimidjian, Martell, Addis, Herman-Dunn, & Barlow, 2008), and persons with chronic pain have been shown to experience less opportunities for positive reinforcement in their daily lives (e.g., Ditre et al., 2011; Loyland et al., 2010). Alcohol may facilitate positive reinforcement via direct appetitive subjective effects (e.g., vigor, elation; de Wit, Uhlenhuth, Pierri, & Johanson, 1987), and by increasing opportunities for social interaction. Alcohol users frequently cite social facilitation and camaraderie as motives for drinking (Kuntsche et al., 2005), and it is possible that persons with recurrent pain may be motivated to use alcohol as a means of maintaining social connectivity. Indeed, there is initial evidence that pain may be associated with greater motivation to use alcohol in social situations (Lawton & Simpson, 2009). It is also interesting to note that measures of pain-related disability typically incorporate assessment of pain-related interference in social functioning (Zale et al., 2013). To the extent that alcohol use may provide opportunities for positive reinforcement via social facilitation, it may also contribute to lower perceived disability (e.g., Kim et al., 2013).
Researchers have suggested that motivation to consume alcohol for pain-coping may increase when alternative coping strategies have failed (Lawton & Simpson, 2009). Thus, the availability and effectiveness of strategies for coping with pain may relate to drinking motivation in at least two ways. First, the employment of more adaptive approaches to pain-coping may depend on the degree to which an individual believes that consuming alcohol will sufficiently diminish pain reactivity. That is, drinkers who believe or come to learn that alcohol can provide adequate pain relief may not engage or develop more adaptive coping strategies (e.g., Cooper et al., 1988). Second, drinkers who neglect to utilize pain-coping behaviors or are unsuccessful in their employment of coping to curtail pain could, subsequently, experience increased motivation to drink. This may be especially true for those drinkers who hold strong expectancies that drinking will help them cope with or reduce pain.
Finally, the previously mentioned allostatic load model (Egli et al., 2012) also indicates that pathological functioning of overlapping neural stress and reward systems may increase motivation to consume alcohol in response to pain. Indeed, aversive stimuli can become conditioned cues that increase drug-seeking behavior (Phillips, Stuber, Heien, Wightman, & Carelli, 2003), and pain may come to serve as a cue for alcohol seeking, possibly via stress-induced dysregulation of both neurotransmitters (e.g., dopamine) and neuropeptides (e.g., corticotropin-releasing hormone). For example, the mesolimbic dopamine system has been implicated in the acquisition of conditioned associations between neutral stimuli and drug-related cues (e.g., Weiss & Porrino, 2002), and stress has been shown to enhance mesolimbic dopamine transmission in both humans and animals (Piazza & Le Moal, 1998; Pruessner, Champagne, Meaney, & Dagher, 2004). Stress-induced dopamine release has been associated with drug seeking among animals (Shaham & Stewart, 1995), and dopamine gene polymorphisms have been associated with stress-induced drug craving among humans (Erblich, Lerman, Self, Diaz, & Bovbjerg, 2004). There is also reason to suspect that corticotropin-releasing factor, a family of related neuropeptides that engender a variety of physiological stress effects, may mediate stress-induced reinstatement of alcohol self-administration (Heilig & Koob, 2007). Corticotropin-releasing hormone, which regulates the release of corticotropin from the pituitary gland, is widely distributed throughout the brain, and has been shown to play a key role in affect regulation and behavioral responses to stressors (Heinrichs & Koob, 2004).
Integrative Conceptualization of Bi-Directional Associations between Pain and Alcohol
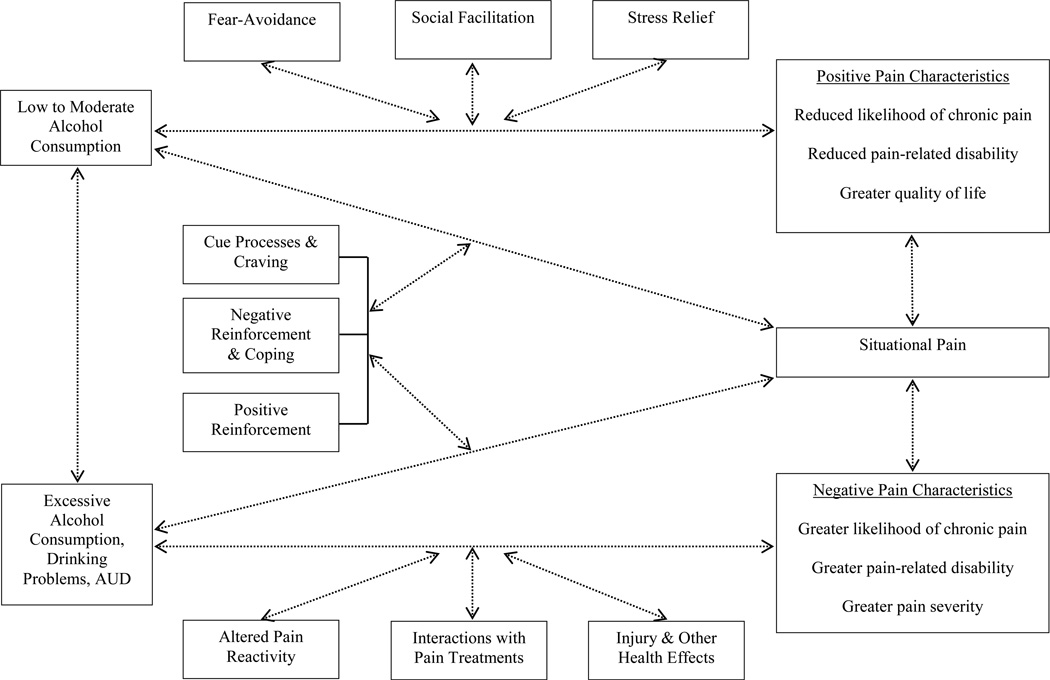
The current review integrated two lines of empirical inquiry (i.e., the effects of alcohol on pain and the effects of pain on alcohol use), with evidence derived from a broad range of epidemiological, clinical, and experimental research. Taken together, these data suggest that pain and alcohol may interact in a bi-directional manner, possibly resulting in greater pain and increased alcohol consumption over time. Accordingly, Figure 3 presents our conceptualization of reciprocal relations between pain and smoking that accounts for varying operational definitions of both alcohol use (e.g., moderate consumption vs. AUD) and pain (e.g., situational/acute vs. chronic) and provides a summary of potential mechanistic factors discussed in the current review. Bi-directional arrows are used to acknowledge that reciprocal influences may occur across associations between pain and alcohol use, and dashed lines are used to illustrate the modest causal evidence derived from the current literature. Dashed, bi-directional lines between moderate and excessive alcohol consumption acknowledge that alcohol use patterns may change (i.e., increase or decrease) over time.
Figure 3.
Conceptualization of bi-directional relations between pain and alcohol use that integrates two lines of empirical inquiry (i.e., effects of alcohol on pain and effects of pain on alcohol use), accounts for varying levels of alcohol consumption, and summarizes potential mechanistic factors identified in the current review.
With regard to the effects of alcohol on pain, moderate alcohol use may be associated with positive pain outcomes, including reduced likelihood of developing chronic pain, and lower levels of pain-related disability and greater quality of life among persons with chronic pain. Hypothesized mechanisms of action include reduced salience of fear-avoidance pathways when drinking, social facilitation effects of alcohol, and stress relieving effects of alcohol. We also reviewed evidence of associations between excessive alcohol use and negative pain outcomes, including an increased likelihood of developing or worsening chronic pain. Hypothesized mechanisms include alterations in pain reactivity (e.g., dysregulation of the endogenous opioid system), adverse interactions with pain medications, and other health effects of alcohol (e.g., increased risk of injury). With regard to the effects of pain on alcohol use, the current review suggests that pain may serve as a situational motivator of alcohol consumption. Indeed, alcohol users may be motivated to drink in response to pain due to both negative (e.g., negative affect, stress, or craving reduction) and positive (e.g., direct appetitive effects of alcohol in the absence of other available reinforcers) reinforcement pathways.
Future Research Directions
Prevalence and Factors Common to Pain and Alcohol Use
Studies utilizing representative population-based and clinical samples are needed to generate prevalence estimates that account for varying definitions of pain (e.g., chronic pain duration, type of pain condition) and alcohol use (e.g., amount consumed, AUD). Future research would benefit from a more detailed and consistent approach to the quantification and operationalization of self-reported alcohol consumption. Future research should also attempt to differentiate between lifetime abstainers and those who abstain later in life (e.g., due to illness that prohibits alcohol use or recovery from AUD), as pain-related outcomes may vary as a function of alcohol exposure. The current review also identified numerous sociodemographic, psychiatric, and environmental factors common to both pain and alcohol use (e.g., socioeconomic status, anxiety and depressive disorders, tobacco smoking). As these factors may confound the study of relations between pain and alcohol, future research would benefit from accounting for these relevant third variables. Future research may also examine other relevant third variables (e.g., comorbid medical conditions, emotional distress) that could further inform our evolving conceptualization of reciprocal relations between pain and alcohol use.
Longitudinal Associations between Pain and Alcohol Use
Limited prospective evidence indicates that, although moderate alcohol use may confer some pain-related benefits, excessive alcohol consumption may be associated with the onset and progression of pain. Additional longitudinal studies are needed to establish the temporal precedence of alcohol consumption and the development/exacerbation of chronic pain. For example, future studies may test prospective associations between varying levels of alcohol consumption and the onset of chronic pain conditions in the general population, as well as associations between varying levels of alcohol use and the progression of chronic pain conditions among treatment-seeking pain patients. Given the hypothesized curvilinear relation between alcohol use and pain outcomes, we recommend quantifying alcohol consumption in a manner that allows for modeling of curvilinear relations (e.g., as a continuous variable).
To our knowledge, all of the studies that examined prospective relations between pain and subsequent alcohol consumption were limited to samples consisting of older adults who reported low levels of baseline drinking and did not necessarily have chronic pain (Bobo et al., 2012; Brennan et al., 2011; Brennan & Soohoo, 2013). Future work in this area should test relations between pain and subsequent patterns of alcohol consumption using representative samples drawn from the general population, treatment-seeking chronic pain patients, and persons seeking treatment for AUD. For example, longitudinal studies may test whether persons with chronic pain are at greater risk for the development or persistence of AUD. Given evidence that pain may motivate alcohol consumption, researchers have hypothesized that recurring pain may increase alcohol consumption over time (Egli et al., 2012), and further research is needed to identify pain-related factors that may contribute to this transition (e.g., pain frequency, pain duration, affective disturbance). Additionally, researchers have suggested that persons who utilize effective pain coping strategies may experience reduced pain and pain-related distress, which, in turn, could result in decreased motivation to use substances (Ditre, Heckman, Butts, & Brandon, 2010). Future research should test whether engagement of effective pain-coping strategies (e.g., in the context of pain treatment) decreases motivation to drink alcohol over time.
Research designs that allow for naturalistic assessment of covariation between pain and alcohol consumption would yield important information regarding event-level temporal associations. For example, ecological momentary assessment (EMA) may provide an optimal method for assessing such covariation in near-real-time. Indeed, EMA has recently been used to examine covariation between pain and tobacco smoking (Dhingra, Homel, et al., 2013), and future research may determine whether increases in pain tend to precede episodes and quantity of drinking, and whether subsequent alcohol consumption serves to decrease pain and pain-related distress/unpleasantness.
Experimental Investigations of Pain and Alcohol Consumption
Experimental studies should be employed to test causal relations between pain and alcohol use, and to identify underlying mechanisms. First, despite evidence that persons with chronic pain endorse the use of alcohol to cope with pain, we are not aware of any experimental studies that have explicitly tested whether pain may motivate alcohol approach or consumption. Future experimental research should test whether situational pain increases craving for alcohol or subsequent alcohol consumption. Second, relatively few experimental studies have been conducted to test effects of alcohol on pain responding. Although previous research has observed variations in analgesic effects across participant samples and alcohol doses, future research would benefit from examining potential mediators/moderators of alcohol-induced analgesia among humans. For example, we are not aware of any studies that have tested whether alcohol-related expectancies for pain relief and/or stress reduction may influence the magnitude of acute analgesic effects, and future research should also attempt to disentangle pharmacologic and expectancy effects (e.g., via use of a balanced-placebo design). Indeed, a better under understanding of mechanisms by which persons in pain may be motivated to consume alcohol (e.g., expectancies for pain relief) would inform the development of tailored interventions. Third, given evidence from animal studies that acute pain-inhibitory effects may subside with chronic alcohol exposure, human trials are also warranted to determine whether tolerance to alcohol reduces acute pain-inhibitory effects. Finally, studies that have examined pain-inhibitory effects of oral alcohol administration typically utilized beverages containing sugar to conceal alcohol dosage (e.g., Brown & Cutter, 1977). Given recent evidence that sugar may reduce pain (Stevens, Yamada, Lee, & Ohlsson, 2013) and amplify the pain-inhibitory effects of other substances (Kanarek & Carrington, 2004), future research should attempt to disentangle the pain mitigating effects of alcohol relative to other constituents (e.g., sucrose).
We also reviewed evidence that persons seeking treatment for AUD demonstrated hyperalgesia to a pain induction task during the initial stages of alcohol abstinence (Jochum et al., 2010). Although this finding is consistent with evidence of abstinence-induced hyperalgesia derived from animal models, additional research among humans is sorely needed. For example, future work in this area could utilize within-subjects designs to assess pain responding prior to and throughout the early stages of alcohol abstinence among persons who present to treatment for AUD. Whenever possible given ethical considerations, experimental studies may also test whether persons with AUD, randomized to varying periods of alcohol abstinence (e.g., 12 hours, 24 hours), demonstrate greater laboratory pain reactivity. Finally, research in the emerging area of pain and alcohol will benefit from experimental investigations that allow for causal inferences and tests of hypothesized mechanisms of action (e.g., negative affect, expectancies for alcohol-induced pain reduction).
Pain and Alcohol Treatment Outcomes
Given evidence that chronic alcohol use may alter pain processing, and that excessive alcohol use may be associated with the development and progression of chronic pain, it seems intuitive to suggest that excessive alcohol consumption may adversely impact pain treatment. However, we are not aware of any studies that examined the effects of excessive or problematic alcohol use on pain treatment outcomes. Core outcomes of pain treatment include pain characteristics (e.g., intensity, opioid use), physical functioning, emotional functioning, and patient disposition (e.g., treatment drop-out; Dworkin et al., 2005). Future studies are needed to identify the unique needs of alcohol users who enter pain treatment. For example, researchers should test whether excessive alcohol consumption precludes pain treatment, reduces treatment efficacy, or interferes with opioid tapering. Additionally, extrapolating from a larger literature on pain and substance use (e.g., Ditre et al., 2011), we hypothesize that chronic or recurrent pain may serve as a barrier to alcohol abstinence and negatively affect alcohol treatment outcomes (e.g., increased treatment drop-out, relapse). Additional studies are needed to test the effects of pain on treatment for AUD or problematic drinking.
Researchers have noted a need for integrated treatments that are informed by knowledge of reciprocal relations between pain and substance use, and initial pilot data suggest that integrated treatments may be beneficial for treating co-occurring pain and substance use disorders (Ilgen et al., 2011). Future work is needed to develop and test integrated interventions for pain and alcohol use across a range of health-care settings. For example, persons with co-occurring pain disorders who engage in treatment for AUD may benefit from taking additional measures to manage their pain during the early stages of alcohol abstinence. Similarly, patients receiving pain treatment may benefit from interventions that seek to reduce the use of alcohol for pain-coping. Finally, given that pain motivates more than half of all annual physician visits in the United States (Mayo Clinic, 2001; Turk & Melzack, 2011), and considering that brief interventions have been shown to improve both pain- (e.g., decreased pain severity and disability; Hay et al., 2005), and alcohol-related outcomes (e.g., reduced alcohol consumption; Kaner et al., 2007), patients who present to primary care with co-occurring pain and excessive alcohol use may benefit from an integrated treatment delivered in that setting.
Summary and Conclusions
The current review integrated evidence of associations between pain and alcohol use derived from a broad range of psychological, social, and biological literatures. Previous work has demonstrated that pain and AUD share overlapping neural circuitry, and that pathological functioning may contribute to the development and maintenance of comorbid chronic pain and alcohol dependence (Egli et al., 2012). The current review builds upon this work by examining associations between pain and varying levels of alcohol consumption, and by proposing additional psychosocial mechanisms that may underlie pain-alcohol interrelations (e.g., fear-avoidance pathways). Specifically, we observed evidence that relations between alcohol use and chronic pain outcomes may be curvilinear in nature (see Figure 2), such that moderate alcohol consumption (vs. no use) may be associated with positive chronic pain outcomes (e.g., decreased disability among persons in pain), and excessive alcohol use, problem drinking, and AUD may be associated with deleterious chronic pain outcomes (e.g., greater pain intensity and interference). We also reviewed evidence that pain may serve as a situational motivator of alcohol consumption. Figure 3 presents our conceptualization of relations between pain and varying levels of alcohol use as being reciprocal in nature, and includes a summary of psychosocial mechanisms that may underlie associations between pain and both low-to-moderate alcohol consumption (e.g., social facilitation) and excessive drinking or AUD (e.g., abstinence-induced hyperalgesia). Finally, we proposed several directions for future research, as informed by our evolving conceptualization of pain and alcohol, to help advance knowledge in this emerging domain.
Supplementary Material
Highlights.
We present an integrative conceptualization of bi-directional pain-alcohol relations
Moderate alcohol use may be associated with positive pain-related outcomes
Excessive drinking/alcohol use disorder may be associated with negative pain outcomes
Alcohol administration may confer acute pain-inhibitory effects
Situational pain may motivate alcohol consumption (e.g., drinking for pain-coping)
Acknowledgments
Role of Funding Source
This work was supported in part by grant R21DA034285 awarded to Joseph W. Ditre by the National Institute on Drug Abuse. NIDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors of this review are Emily L. Zale, Stephen A. Maisto, and Joseph W. Ditre. All authors have contributed significantly to this manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–1140. doi: 10.1016/s0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- Ang DC, Peloso PM, Woolson RF, Kroenke K, Doebbeling BN. Predictors of incident chronic widespread pain among veterans following the first Gulf War. Clinical Journal of Pain. 2006;22:554–563. doi: 10.1097/01.ajp.0000208907.42506.21. [DOI] [PubMed] [Google Scholar]
- Arcury TA, Bernard SL, Jordan JM, Cook HL. Gender and ethnic differences in alternative and conventional arthritis remedy use among community-dwelling rural adults with arthritis. Arthritis Care and Research. 1996;9:384–390. doi: 10.1002/1529-0131(199610)9:5<384::aid-anr1790090507>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Armeli S, Carney MA, Tennen H, Affleck G, O'Neil TP. Stress and alcohol use: a daily process examination of the stressor-vulnerability model. Journal of Personality and Social Psychology. 2000;78:979–994. doi: 10.1037//0022-3514.78.5.979. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Research and Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. Journal of Rheumatology. 2002;29:818–825. [PubMed] [Google Scholar]
- Bien TH, Burge R. Smoking and drinking: a review of the literature. International Journal of the Addictions. 1990;25:1429–1454. doi: 10.3109/10826089009056229. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? Journal of Neuroendocrinology. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Greek AA, Klepinger DH, Herting JR. Predicting 10-Year Alcohol Use Trajectories Among Men Age 50 Years and Older. American Journal of Geriatric Psychiatry. 2012 doi: 10.1016/j.jagp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Booker EA, Haig AJ, Geisser ME, Yamakawa K. Alcohol use self report in chronic back pain--relationships to psychosocial factors, function performance, and medication use. Disability and Rehabilitation. 2003;25:1271–1277. doi: 10.1080/09638280310001608609. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Veltman DJ, Beekman AT, Penninx BW. Alcohol use disorders and the course of depressive and anxiety disorders. British Journal of Psychiatry. 2012;200:476–484. doi: 10.1192/bjp.bp.111.097550. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol and Alcoholism. 2004;39:357–361. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Braden JB, Zhang L, Zimmerman FJ, Sullivan MD. Employment outcomes of persons with a mental disorder and comorbid chronic pain. Psychiatric Services. 2008;59:878–885. doi: 10.1176/appi.ps.59.8.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, SooHoo S, Moos RH. Painful medical conditions and alcohol use: a prospective study among older adults. Pain Medicine. 2011;12:1049–1059. doi: 10.1111/j.1526-4637.2011.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Soohoo S. Pain and use of alcohol in later life: prospective evidence from the health and retirement study. Journal of Aging and Health. 2013;25:656–677. doi: 10.1177/0898264313484058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Cutter HS. Alcohol, customary drinking behavior, and pain. Journal of Abnormal Psychology. 1977;86:179–188. doi: 10.1037/0021-843X.86.2.179. [DOI] [PubMed] [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, Saxon AJ. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction. 2008;103:1996–2005. doi: 10.1111/j.1360-0443.2008.02358.x. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcoholism, Clinical and Experimental Research. 2007;31:1435–1440. doi: 10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ, Group LS. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- CDC. About BMI for Adults. 2011 from http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
- CDC. Alcohol Related Disease Impact (ARDI) application. 2012a Retrieved 8/27/13, from http://apps.nccd.cdc.gov/DACH_ARDI/Default.aspx.
- CDC. Current cigarette smoking among adults - United States 2011. Morbidity and Mortality Weekly Report. 2012b;61:889–894. [PubMed] [Google Scholar]
- Chartier K, Caetano R. Ethnicity and Health Disparities in Alcohol Research. Alcohol Res Health. 2010;33:152–160. [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? Journal of Clinical Epidemiology. 2000;53:315–322. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- Chopra K, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. British Journal of Clinical Pharmacology. 2012;73:348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: a test of social learning formulations. Journal of Abnormal Psychology. 1988;97:218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- Cutter HS, Jones WC, Maloof BA, Kurtz NR. Pain as a joint function of alcohol intake and customary reasons for drinking. International Journal of the Addictions. 1979;14:173–182. doi: 10.3109/10826087909060363. [DOI] [PubMed] [Google Scholar]
- Cutter HS, Maloof B, Kurtz NR, Jones WC. "Feeling no pain" differential responses to pain by alcoholics and nonalcoholics before and after drinking. Journal of Studies on Alcohol. 1976;37:273–277. doi: 10.15288/jsa.1976.37.273. [DOI] [PubMed] [Google Scholar]
- Dawson DA. US low-risk drinking guidelines: an examination of four alternatives. Alcoholism, Clinical and Experimental Research. 2000;24:1820–1829. [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Prospective correlates of drinking cessation: variation across the life-course. Addiction. 2013;108:712–722. doi: 10.1111/add.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual Differences in Behavioral and Subjective Responses to Alcohol. Alcoholism: Clinical and Experimental Research. 1987;11:52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosomatic Medicine. 2002;64:773–786. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- Dhingra LK, Homel P, Grossman B, Chen J, Scharaga E, Calamita S, Portenoy R. Ecological Momentary Assessment of Smoking Behavior in Persistent Pain Patients. Clinical Journal of Pain. 2013 doi: 10.1097/AJP.0b013e31829821c7. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Martell CR, Addis ME, Herman-Dunn R, Barlow DH. Clinical handbook of psychological disorders: A step-by-step treatment manual. 4th ed. New York, NY US: Guilford Press; 2008. Behavioral activation for depression; pp. 328–364. [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin. 2011;137:1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology. 2010;119:524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JE, Molina BS, Kelly TM. Alcohol outcome expectancies as socially shared and socialized beliefs. Psychology of Addictive Behaviors. 2009;23:248–259. doi: 10.1037/a0015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27:286–290. doi: 10.1097/00006676-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65:437–443. doi: 10.1212/01.wnl.0000171862.17301.84. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neuroscience and Biobehavioral Reviews. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Walter NAR, Dick DM, Buck KJ, Crabbe JC. Review: A comparison of selected quantitative trait loci associated with alcohol use phenotypes in humans and mouse models. Addiction Biology. 2010;15:185–199. doi: 10.1111/j.1369-1600.2009.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics Journal. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- Esper LH, Furtado EF. Gender differences and association between psychological stress and alcohol consumption: A systematic review. Journal of Alcoholism and Drug Dependence. 2013;1:116–121. [Google Scholar]
- FDA. Department of Health and Human Services. Rockville, MD: 1998. Over-the-Counter Drug Products Containing Analgesic/Antipyretic Active Ingredients for Internal Use; Required Alcohol Warning. Retrieved from http://www.fda.gov/ohrms/dockets/98fr/102398d.txt. [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JW, Moos RH, Brennan PL. The Drinking Problems Index: a measure to assess alcohol-related problems among older adults. Journal of Substance Abuse. 1991;3:395–404. doi: 10.1016/s0899-3289(10)80021-5. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Lewis JE, Cole B, Cutler RB, Rosomoff HL, Rosomoff RS. Variables associated with current smoking status in chronic pain patients. Pain Medicine. 2007;8:301–311. doi: 10.1111/j.1526-4637.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- Fraser AG. Pharmacokinetic interactions between alcohol and other drugs. Clinical Pharmacokinetics. 1997;33:79–90. doi: 10.2165/00003088-199733020-00001. [DOI] [PubMed] [Google Scholar]
- Gatch MB. Effects of benzodiazepines on acute and chronic ethanol-induced nociception in rats. Alcoholism, Clinical and Experimental Research. 1999;23:1736–1743. [PubMed] [Google Scholar]
- Gatch MB. Ethanol withdrawal and hyperalgesia. Current Drug Abuse Reviews. 2009;2:41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Lal H. Effects of Ethanol and Ethanol Withdrawal on Nociception in Rats. Alcoholism: Clinical and Experimental Research. 1999;23:328–333. [PubMed] [Google Scholar]
- Gatch MB, Selvig M. Theophylline blocks ethanol withdrawal-induced hyperalgesia. Alcohol and Alcoholism. 2002;37:313–317. doi: 10.1093/alcalc/37.4.313. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. Journal of Psychiatry and Neuroscience. 2001;26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Goebel JR, Compton P, Zubkoff L, Lanto A, Asch SM, Sherbourne CD, Lorenz KA. Prescription sharing, alcohol use, and street drug use to manage pain among veterans. Journal of Pain and Symptom Management. 2011;41:848–858. doi: 10.1016/j.jpainsymman.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Gorman DM, Potamianos G, Williams KA, Frank AO, Duffy SW, Peters TJ. Relationship between alcohol abuse and low back pain. Alcohol and Alcoholism. 1987;22:61–63. [PubMed] [Google Scholar]
- Govindu NK, Babski-Reeves K. Effects of personal, psychosocial and occupational factors on low back pain severity in workers. International Journal of Industrial Ergonomics. 2013 [Google Scholar]
- Green CR, Hart-Johnson T. The association between race and neighborhood socioeconomic status in younger Black and White adults with chronic pain. Journal of Pain. 2012;13:176–186. doi: 10.1016/j.jpain.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Gureji O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A World Health Organization Study in Primary Care. JAMA. 1997;280:147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hay EM, Mullis R, Lewis M, Vohora K, Main CJ, Watson P, Croft PR. Comparison of physical treatments versus a brief pain-management programme for back pain in primary care: a randomised clinical trial in physiotherapy practice. Lancet. 2005;365:2024–2030. doi: 10.1016/S0140-6736(05)66696-2. [DOI] [PubMed] [Google Scholar]
- He L, Whistler JL. Chronic ethanol consumption in rats produces opioid antinociceptive tolerance through inhibition of mu opioid receptor endocytosis. PloS One. 2011;6:e19372. doi: 10.1371/journal.pone.0019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. Journal of Pharmacology and Experimental Therapeutics. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Helmer DA, Chandler HK, Quigley KS, Blatt M, Teichman R, Lange G. Chronic widespread pain, mental health, and physical role function in OEF/OIF veterans. Pain Medicine. 2009;10:1174–1182. doi: 10.1111/j.1526-4637.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clinical Therapeutics. 2007;29(Suppl):2477–2497. doi: 10.1016/j.clinthera.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Hestbaek L, Leboeuf-Yde C, Kyvik KO. Are lifestyle-factors in adolescence predictors for adult low back pain? A cross-sectional and prospective study of young twins. BMC Musculoskeletal Disorders. 2006;7:27. doi: 10.1186/1471-2474-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuch I, Hagen K, Zwart JA. Body mass index as a risk factor for developing chronic low back pain: a follow-up in the Nord-Trondelag Health Study. Spine (Phila Pa 1976) 2013;38:133–139. doi: 10.1097/BRS.0b013e3182647af2. [DOI] [PubMed] [Google Scholar]
- Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult: family linkage data from the HUNT Study. JAMA Pediatrics. 2013;167:61–69. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Cronkite RC, Randall PK. Drinking to cope, emotional distress and alcohol use and abuse: a ten-year model. Journal of Studies on Alcohol. 2001;62:190–198. doi: 10.15288/jsa.2001.62.190. [DOI] [PubMed] [Google Scholar]
- Holmes A, Williamson O, Hogg M, Arnold C, Prosser A, Clements J, O'Donnell M. Predictors of pain severity 3 months after serious injury. Pain Medicine. 2010;11:990–1000. doi: 10.1111/j.1526-4637.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152:223–229. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckle T, You RQ, Casswell S. Socio-economic status predicts drinking patterns but not alcohol-related consequences independently. Addiction. 2010;105:1192–1202. doi: 10.1111/j.1360-0443.2010.02931.x. [DOI] [PubMed] [Google Scholar]
- IASP. Part III: Pain Terms, A Current List with Definitions and Notes on Usage. In: Merskey N HB, editor. Classification of Chronic Pain, Second Edition. Seattle: IASP Press; 1994. [Google Scholar]
- Ibironke GF, Oyekunle OA. Ethanol-Induced Antinociception in Rodents: Role of the Cholinergic and Opioidergic Systems. Neurophysiology. 2012;44:460–463. [Google Scholar]
- Ilgen MA, Haas E, Czyz E, Webster L, Sorrell JT, Chermack S. Treating Chronic Pain in Veterans Presenting to an Addictions Treatment Program. Cognitive and Behavioral Practice. 2011;18:149–160. [Google Scholar]
- Ilgen MA, McLouth C, Barry KL, Walton M, Cole PA, Dabrowski MP, Blow FC. Pain interference in individuals in driver intervention programs for driving under the influence offenders. Substance Use and Misuse. 2010;45:1406–1419. doi: 10.3109/10826081003682818. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Zivin K, McCammon RJ, Valenstein M. Pain and suicidal thoughts, plans and attempts in the United States. General Hospital Psychiatry. 2008;30:521–527. doi: 10.1016/j.genhosppsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen S, Valvanne J, Pitkala KH. Alcohol use of older adults: drinking alcohol for medicinal purposes. Age and Ageing. 2011;40:633–637. doi: 10.1093/ageing/afr089. [DOI] [PubMed] [Google Scholar]
- IOM. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- James MF, Duthie AM, Duffy BL, McKeag AM, Rice CP. Analgesic effect of ethyl alcohol. British Journal of Anaesthesia. 1978;50:139–141. doi: 10.1093/bja/50.2.139. [DOI] [PubMed] [Google Scholar]
- Jobski K, Schmid U, Behr S, Andersohn F, Garbe E. 3-year prevalence of alcohol-related disorders in German patients treated with high-potency opioids. Pharmacoepidemiology and Drug Safety. 2012;21:1125–1129. doi: 10.1002/pds.3268. [DOI] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bar KJ. Increased pain sensitivity in alcohol withdrawal syndrome. European Journal of Pain. 2010;14:713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Carrington C. Sucrose consumption enhances the analgesic effects of cigarette smoking in male and female smokers. Psychopharmacology. 2004;173:57–63. doi: 10.1007/s00213-003-1699-0. [DOI] [PubMed] [Google Scholar]
- Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, Burnand B. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database of Systematic Reviews. 2007:CD004148. doi: 10.1002/14651858.CD004148.pub3. [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis and Rheumatism. 2006;54:1682–1686. doi: 10.1002/art.21798. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kim CH, Vincent A, Clauw DJ, Luedtke CA, Thompson JM, Schneekloth TD, Oh TH. Association between alcohol consumption and symptom severity and quality of life in patients with fibromyalgia. Arthritis Research & Therapy. 2013;15:R42. doi: 10.1186/ar4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Hirota Y, Kawamura H, Miura H, Takasugi S, Sugioka Y, Iwamoto Y. Factors associated with pain and functional limitation in Japanese male patients with knee osteoarthritis. Rheumatology International. 2007;27:1135–1142. doi: 10.1007/s00296-007-0356-z. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clinical Psychology Review. 2005;25:841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102:752–760. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- Lawton J, Simpson J. Predictors of alcohol use among people experiencing chronic pain. Psychology, Health & Medicine. 2009;14:487–501. doi: 10.1080/13548500902923177. [DOI] [PubMed] [Google Scholar]
- Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of Behavioral Medicine. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- Lerch MM, Albrecht E, Ruthenburger M, Mayerle J, Halangk W, Kruger B. Pathophysiology of alcohol-induced pancreatitis. Pancreas. 2003;27:291–296. doi: 10.1097/00006676-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Loyland B, Miaskowski C, Paul SM, Dahl E, Rustoen T. The relationship between chronic pain and health-related quality of life in long-term social assistance recipients in Norway. Quality of Life Research. 2010;19:1457–1465. doi: 10.1007/s11136-010-9707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Cash KA, Damron KS, Manchukonda R, Pampati V, McManus CD. Controlled substance abuse and illicit drug use in chronic pain patients: An evaluation of multiple variables. Pain physician. 2006;9:215–225. [PubMed] [Google Scholar]
- Marcus DA. Obesity and the impact of chronic pain. Clinical Journal of Pain. 2004;20:186–191. doi: 10.1097/00002508-200405000-00009. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic. Managing Pain: Attitude, Medication and therapy are keys to control. Mayo Clinic Health Letter. 2001 http://mayoclinic.com.
- McCaffrey RJ, Duff K, Westervelt HJ, editors. Practitioner's guide to evaluating change with neurophsychological assessment instruments. New York, NY: Kluwer Academic/Plenum Publishers; 2000. [Google Scholar]
- McCarthy DE, Curtin JJ, Piper ME, Baker TB. Negative Reinforcement. In: Kassel J, editor. Substance Abuse and Emotion. Washington, D.C: American Psychological Association; 2010. pp. 16–42. [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain and Symptom Management. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol Consumption Induces Endogenous Opioid Release in the Human Orbitofrontal Cortex and Nucleus Accumbens. Science Translational Medicine. 2012;4:116. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mullady DK, Yadav D, Amann ST, O'Connell MR, Barmada MM, Elta GH, Consortium N. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. doi: 10.1136/gut.2010.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njamnshi A, Wisysonge C. Treatment for alcohol-related neuropathy. Chochrane Database of Systematic Reviews. 2010 [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Panconesi A. Alcohol and migraine: trigger factor, consumption, mechanisms. A review. Journal of Headache and Pain. 2008;9:19–27. doi: 10.1007/s10194-008-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecukonis EV. Female children of alcoholics and chronic back pain. Pain Medicine. 2004;5:196–201. doi: 10.1111/j.1526-4637.2004.04024.x. [DOI] [PubMed] [Google Scholar]
- Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug and Alcohol Dependence. 2000;60:221–247. doi: 10.1016/s0376-8716(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Pergolizzi JV, Jr., Gharibo C, Passik S, Labhsetwar S, Taylor R, Jr., Pergolizzi JS, Muller-Schwefe G. Dynamic risk factors in the misuse of opioid analgesics. Journal of Psychosomatic Research. 2012;72:443–451. doi: 10.1016/j.jpsychores.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Perrino AC, Jr., Ralevski E, Acampora G, Edgecombe J, Limoncelli D, Petrakis IL. Ethanol and pain sensitivity: effects in healthy subjects using an acute pain paradigm. Alcoholism, Clinical and Experimental Research. 2008;32:952–958. doi: 10.1111/j.1530-0277.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic Medicine. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in Pharmacological Sciences. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Plunk AD, Syed-Mohammed H, Cavazos-Rehg P, Bierut LJ, Grucza RA. Alcohol Consumption, Heavy Drinking, and Mortality: Rethinking the J-Shaped Curve. Alcoholism: Clinical and Experimental Research. 2013 doi: 10.1111/acer.12250. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Perrino A, Acampora G, Koretski J, Limoncelli D, Petrakis I. Analgesic effects of ethanol are influenced by family history of alcoholism and neuroticism. Alcoholism: Clinicaland Experimental Research. 2010;34:1433–1441. doi: 10.1111/j.1530-0277.2010.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, 3rd, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. Journal of Pain. 2009;10:944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, 3rd, Gilbert GH, Heft MW. Orofacial pain: racial and sex differences among older adults. Journal of Public Health Dentistry. 2002;62:132–139. doi: 10.1111/j.1752-7325.2002.tb03434.x. [DOI] [PubMed] [Google Scholar]
- Rivara FP, Mackenzie EJ, Jurkovich GJ, Nathens AB, Wang J, Scharfstein DO. Prevalence of pain in patients 1 year after major trauma. Archives of Surgery. 2008;143:282–287. doi: 10.1001/archsurg.2007.61. [DOI] [PubMed] [Google Scholar]
- Sa KN, Baptista AF, Matos MA, Lessa I. Chronic pain and gender in Salvador population, Brazil. Pain. 2008;139:498–506. doi: 10.1016/j.pain.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Sá KN, de Mesquita Pereira C, Souza RC, Baptista AF, Lessa I. Knee Pain Prevalence and Associated Factors in a Brazilian Population Study. Pain Medicine. 2011;12:394–402. doi: 10.1111/j.1526-4637.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- Saffier K, Colombo C, Brown D, Mundt MP, Fleming MF. Addiction Severity Index in a chronic pain sample receiving opioid therapy. Journal of Substance Abuse Treatment. 2007;33:303–311. doi: 10.1016/j.jsat.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Drug abuse warning network, 2010: National estimates of drug-related emergency deaprtment visits. Rockville, MD: 2012. [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Moreland RL. Alcohol and group formation: a multimodal investigation of the effects of alcohol on emotion and social bonding. Psychological Science. 2012;23:869–878. doi: 10.1177/0956797611435134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JS, Lucas JW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2011. National Center for Helath Statistics. Vital Health Stat. 2012;10 [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Hesselbrock V, Bucholz KK, Bierut L, Edenberg H, Trim R. Clinical Implications of Tolerance to Alcohol in Nondependent Young Drinkers. American Journal of Drug and Alcohol Abuse. 2008;34:133–149. doi: 10.1080/00952990701877003. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaw BA, Krause N, Liang J, McGeever K. Age differences in long-term patterns of change in alcohol consumption among aging adults. Journal of Aging and Health. 2011;23:207–227. doi: 10.1177/0898264310381276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy CN, Cady RK. Historical perspective of pain management. In: Weiner RS, editor. Pain Management: A practical guide for clinicians. 6th ed. Boca Raton, FL: CRC Press LLC; 2002. pp. 9–16. [Google Scholar]
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Medicine. 2008;9:911–917. doi: 10.1111/j.1526-4637.2008.00420.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillgate E, Vingard E, Josephson M, Holm LW, Alfredsson L. Is smoking and alcohol consumption associated with long-term sick leave due to unspecific back or neck pain among employees in the public sector? Results of a three-year follow-up cohort study. Journal of Rehabilitation Medicine. 2009;41:550–556. doi: 10.2340/16501977-0370. [DOI] [PubMed] [Google Scholar]
- Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews. 2013;1:CD001069. doi: 10.1002/14651858.CD001069.pub4. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Finn PR, Pihl RO. A dose-response study of the effects of alcohol on the perceptions of pain and discomfort due to electric shock in men at high familial-genetic risk for alcoholism. Psychopharmacology. 1995;119:261–267. doi: 10.1007/BF02246289. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug and Alcohol Dependence. 2004;73:23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Watanabe M. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity With Depression-Anxiety Disorders. The Journal of Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Turk DC, Melzack R. The measurement of pain and the assessment of people experiencing pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 3rd ed. New York, NY: The Guilford Press; 2011. pp. 3–18. [Google Scholar]
- Turk DC, Okifuji A. Pain terms and taxonomies of pain. In: Loeser JD, Chapman CR, Turk DC, Butler SH, editors. Bonica's management of pain. 3 ed. Hagerstown, MD: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clinical Journal of Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- U.S. Departments of Agriculture and Health and Human Services. Dietary Guidelines for Americans, 2010. 7 ed. Washington, D.C: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M. Assessment of chronic pain in epidemiological and health services research. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment: Third Edition. New York, NY: The Guilford Press; 2011. [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68:157–167. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: Recent advances and challenges. Journal of Neuroscience. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff HG, Hardy JD, Goodell H. Measurement of the Effect on the Pain Threshold of Acetylsalicylic Acid, Acetanilid, Acetophenetidin, Aminopyrine, Ethyl Alcohol, Trichlorethylene, a Barbiturate, Quinine, Ergotamine Tartrate and Caffeine: An Analysis of Their Relation to the Pain Experience. Journal of Clinical Investigation. 1941;20:63–80. doi: 10.1172/JCI101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow KM, Eltherington LG. Feeling no pain: alcohol as an analgesic. Pain. 1988;32:159–163. doi: 10.1016/0304-3959(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Taylor AN. Genetic differences in opiate receptor concentration and sensitivity to ethanol's effects. Pharmacology, Biochemistry and Behavior. 1989;33:793–796. doi: 10.1016/0091-3057(89)90472-3. [DOI] [PubMed] [Google Scholar]
- Zale EL, Lange KL, Fields SA, Ditre JW. The Relation Between Pain-Related Fear and Disability: A Meta-Analysis. Journal of Pain. 2013 doi: 10.1016/j.jpain.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.