Table 1.
Optimization of the stannylation on 1a.
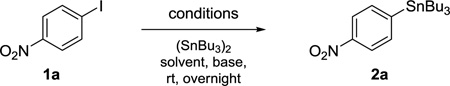 | ||||
|---|---|---|---|---|
| entry | solvent | catalyst (equiv) | base (equiv) | yield (%)c |
| 1a | i-PrOH | Pd(PPh3)4 (0.1) | DIEA (2.5) | <10 |
| 2a | i-PrOH | PdCl2(PPh3)2 (0.1) | DIEA (2.5) | 0 |
| 3a | i-PrOH | PdCl2(PTol3)2 (0.1) | DIEA (2.5) | 19 |
| 4a | i-PrOH | Pd2(dba)3 (0.1) | DIEA (2.5) | 92 |
| 5b | i-PrOH | Pd2(dba)3 (0.025) | DIEA (2.5) | 97 |
| 6b | toluene | Pd2(dba)3 (0.025) | DIEA (2.5) | 37 |
| 7b | THF | Pd2(dba)3 (0.025) | DIEA (2.5) | 44 |
| 8b | hexanes | Pd2(dba)3 (0.025) | DIEA (2.5) | 14 |
| 9b | MeOH | Pd2(dba)3 (0.025) | DIEA (2.5) | 62 |
| 10b | i-PrOH | Pd2(dba)3 (0.025) | DIEA (1.0) | 94 |
| 11b | i-PrOH | Pd2(dba)3 (0.025) | K2CO3 (2.5) | 73 |
| 12b | i-PrOH | Pd2(dba)3 (0.025) | pyridine (2.5) | 0 |
| 13b | i-PrOH | Pd2(dba)3 (0.025) | no base | 68 |
2 Equiv (Bu3Sn)2.
1.1 Equiv (Bu3Sn)2.
Isolated yields.
