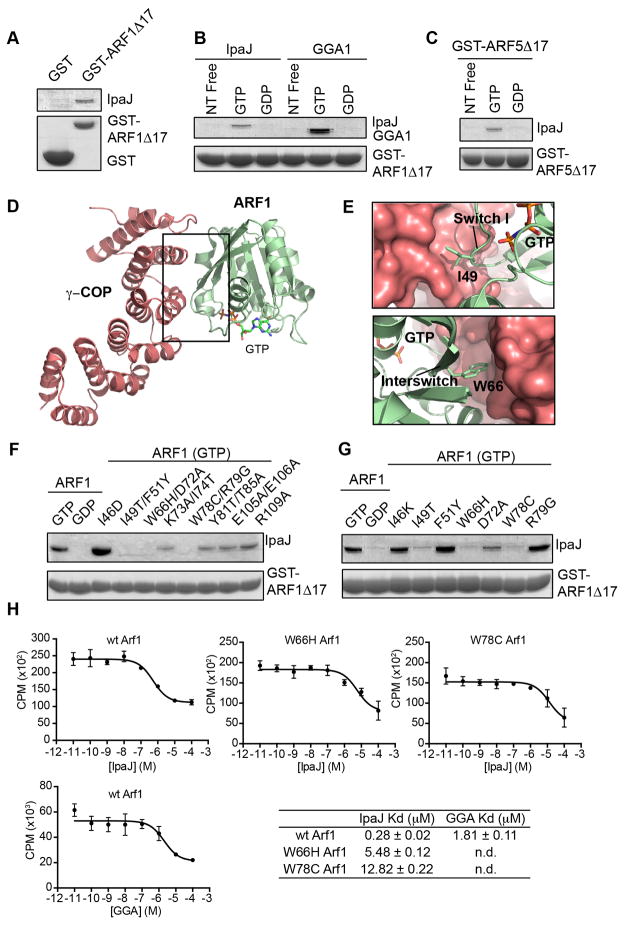
Figure 5. IpaJ functions as an ARF substrate.
(A) Glutathione-pulldown of GST (control) or GST-ARF1Δ17 in the presence of MBP-IpaJΔ50. Proteins by separated with SDS PAGE and visualized by Coomassie staining.
(B) Glutathione-pulldown of GST-ARF1Δ17 in the nucleotide free (free), GTP, or GDP bound state in the presence of MBP-IpaJΔ50 (left) or the GAT domain of GGA1 (right). Proteins were separated by SDS PAGE and visualized by Coomassie staining.
(C) Glutathione-pulldown of GST-ARF5Δ17 in the nucleotide free (free), GTP, or GDP bound state in presence of MBP-IpaJΔ50 (left) and visualized as in (A).
(D and E) Crystal structure of GTP-bound ARF1 (green) in complex with the coatamer subunit gamma-1 (red) (PDB ID: 3TJZ). Binding interface comprises switch I and interswitch regions of ARF1. (E) Specifically, Ile49 and Trp66 (see Figure 5G) participate in this interaction.
(F and G) Glutathione-pulldown of GST-ARF1Δ17 harboring the indicated mutations in the presence of MBP-IpaJΔ50. Proteins were visualized as in (A).
(H) Homologous competition experiments between GTP-loaded ARF1 GTPase domain (ARF1Δ17) and the indicated effector substrates. Dissociation (Kd) constants are shown.