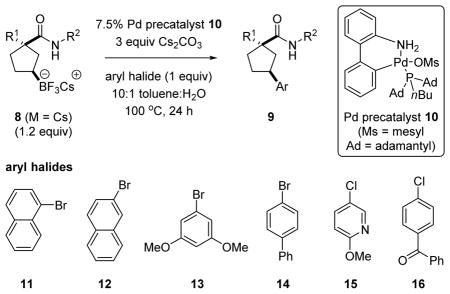
Table 2.
Palladium-catalyzed cross-coupling of cesium tri-fluoroborate salt 8i.
| ||||||
|---|---|---|---|---|---|---|
| entry | 8 | R1 | R2 | aryl halide | 9 | yielda |
| 1b | 8g | Ph | Bn | 11 | a | 69 |
| 2b | 8h | CF3 | Bn | 11 | b | 63 |
| 3 | 8i | Ph | (R)-CH(Me)Ph | 11 | c | 86 |
| 4 | 8i | Ph | (R)-CH(Me)Ph | 12 | d | 66 |
| 5 | 8i | Ph | (R)-CH(Me)Ph | 13 | e | 66 |
| 6 | 8i | Ph | (R)-CH(Me)Ph | 14 | f | 71 |
| 7 | 8i | Ph | (R)-CH(Me)Ph | 15 | g | 75c |
| 8 | 8i | Ph | (R)-CH(Me)Ph | 16 | h | 70 |
Isolated yields based on limiting aryl halide, an average (±2%) of two runs.
Cross-coupling proceeds with high diastereoselectivity (ca. 94:6 dr).
5 equiv of CsOH replaces Cs2CO3.
