Extended Data Figure 4. Purification of a Cas9-Cas1-Cas2-Csn2 complexes.
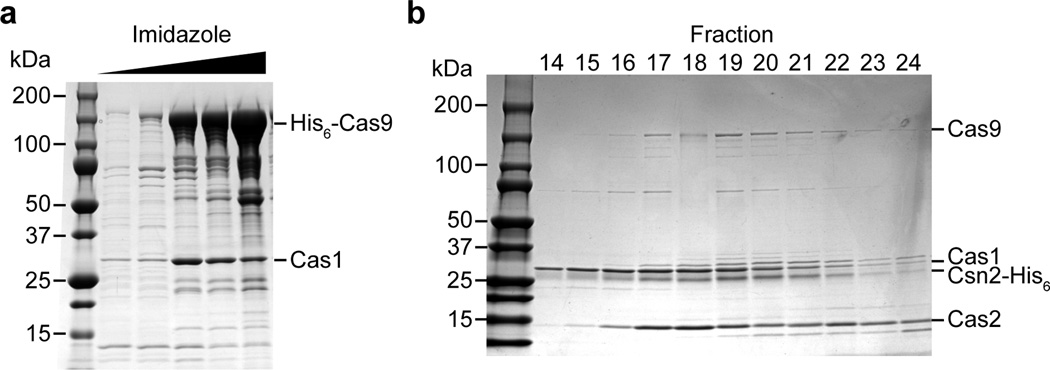
a, The cas9-cas1-cas2-csn2 operon of S. pyogenes SF370 was cloned into the pET16b vector (generating pKW07) to add an N-terminal histidyl tag to Cas9 and express all proteins in E. coli. Purification was performed using Ni-NTA affinity chromatography. SDS-PAGE followed by Coomassie stain of the purified proteins revealed a co-purifying protein that was identified as Cas1 by mass spectrometry, representative of five technical replicates. Mass spectrometry identification of all the eluted proteins co-purifying with Cas9 is shown in Extended Data Table 2. b, The cas9-cas1-cas2-csn2 operon of S. pyogenes SF370 was cloned into the pET23a vector (generating pKW06) to add an C-terminal histidyl tag to Csn2 and express all proteins in E. coli. Purification was performed using Ni-NTA affinity chromatography followed by ion exchange chromatography. The elution fractions that constituted the peak containing the complex (Fig. 3a) were separated by SDS-PAGE and visualized by Coomassie staining, representative of three technical replicates.
