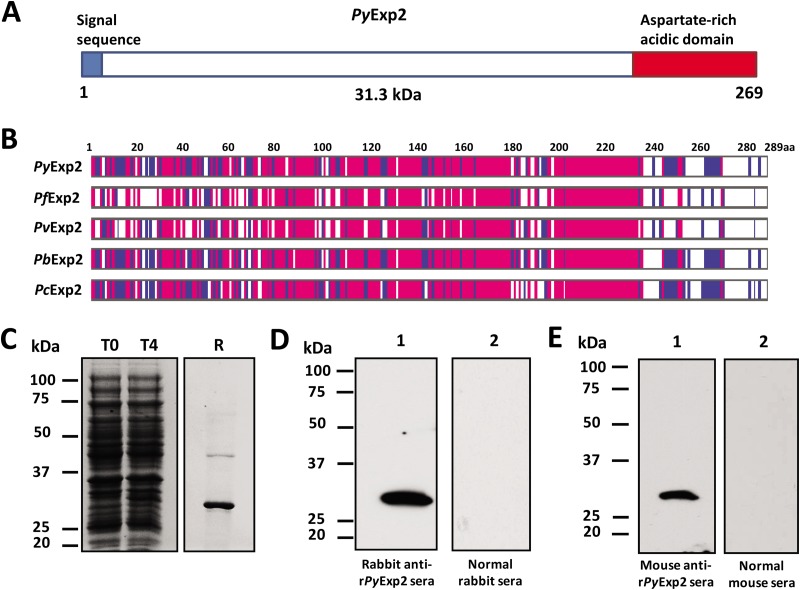
FIG 1.
Expression and purification of rPyExp2. (A) Schematic structure of PyExp2 indicating the N-terminal signal sequence (blue) and the location of the C-terminal charged domain (red). (B) Alignment of the deduced amino acid (aa) sequence of PyExp2 with those of Exp2 proteins in other Plasmodium spp. Blue, identical residues in at least two sequences; pink, identical residues in all five sequences. (C) Coomassie blue-stained SDS-PAGE gel showing E. coli lysate at the time of IPTG induction (T0) and 4 h postinduction (T4) and recombinant protein (R) (3 μg) purified by nickel chelate affinity chromatography. (D) Immunoblot analysis of P. yoelii 17X parasite antigen lysate (5 μg) probed with rabbit anti-rPyExp2 sera (lane 1) or normal rabbit sera (lane 2). (E) Immunoblot analysis of P. yoelii 17X parasite antigen lysate (5 μg) probed with mouse anti-rPyExp2 sera (lane 1) or normal mouse sera (lane 2). Molecular mass markers are indicated.